What is Geistlich Mucograft®?
Geistlich Mucograft® is a unique collagen matrix designed specifically for soft tissue regeneration as an alternative to autogenous soft-tissue grafts.
What is Geistlich Mucograft® made of?
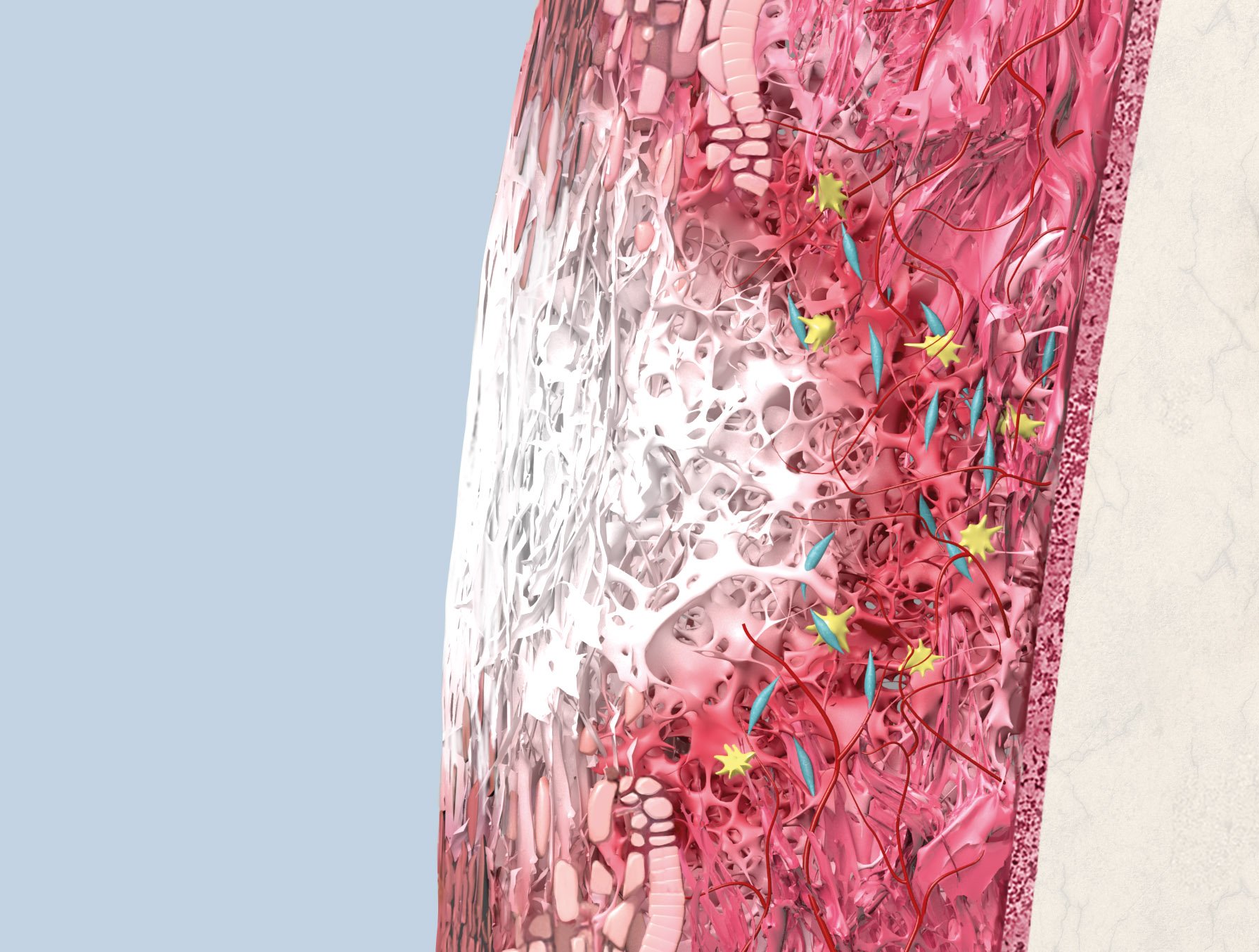
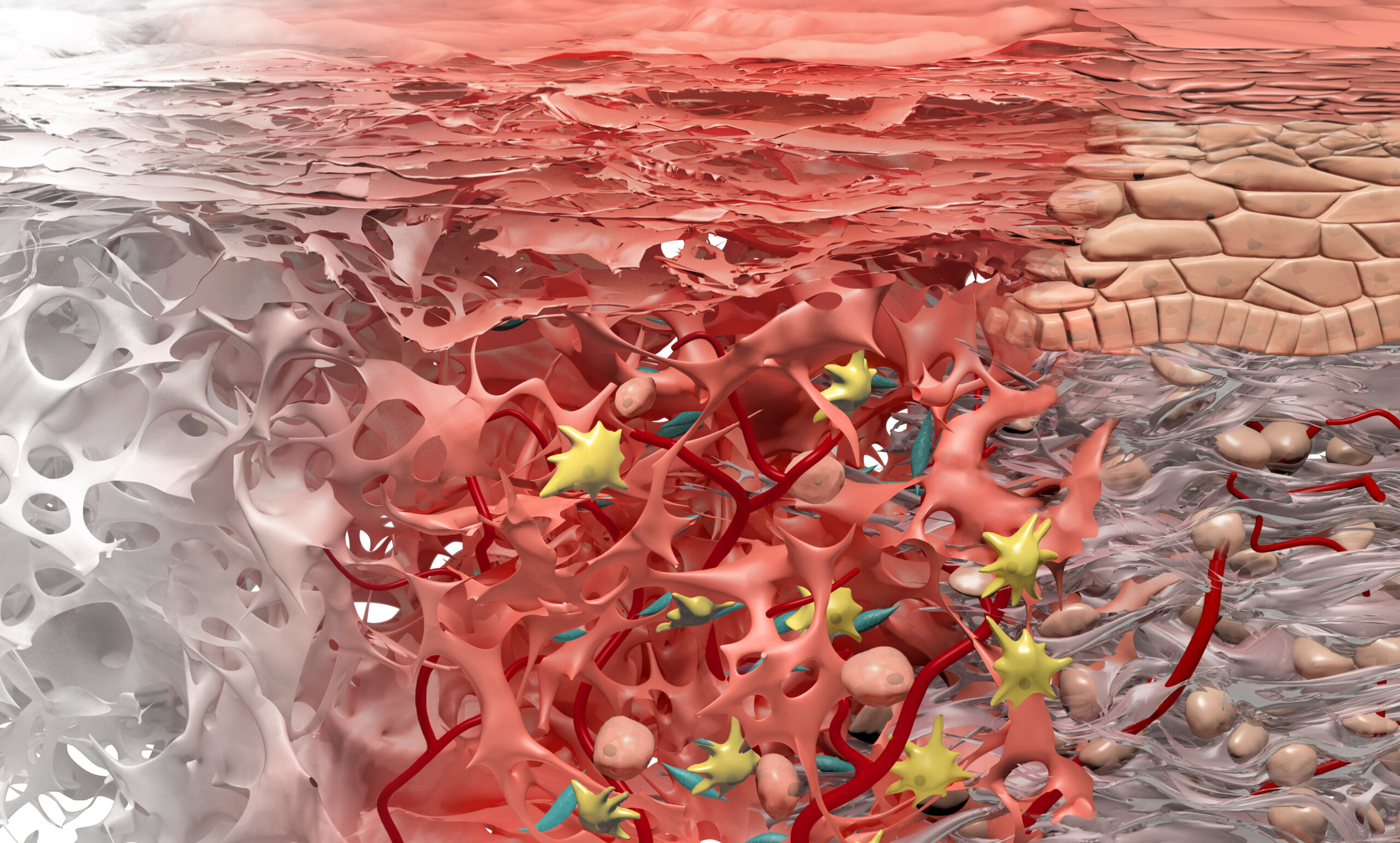
Geistlich Mucograft® consists of porcine collagen and is specifically designed for soft tissue regeneration. The matrix is comprised of a compact structure that provides stability while allowing open healing, paired with a spongy structure that supports blood clot stabilization and ingrowth of soft-tissue cells.
Is Geistlich Mucograft® resorbable?
The collagen in Geistlich Mucograft® is methodically replaced by newly formed soft tissue. The matrix is rapidly vascularized and colonized by soft tissue cells. This leads to a good integration of the matrix without any signs of foreign body reaction1,2.
- Rocchietta I, et al.: Int J Periodontics Restorative Dent 2012; 32(1): e34-40.
- Ghanaati S, et al.: Biomed Mater 2011; 6(1): 015010.
Is Geistlich Mucograft® an alternative for free gingival graft (FGG) or for connective tissue graft (CTG)?
Geistlich Mucograft® can be used as an alternative to both a free gingival graft or a connective tissue graft (CTG) for increasing keratinized tissue1-3. Geistlich Mucograft® is also an alternative to CTG for use in recession coverage procedures4-6.
- Sanz M, et al.: J Clin Periodontol 2009; 36(10): 868-76.
- Lorenzo R, et al.: Clin. Oral Impl. Res 2012; 23(3): 316-24.
- Nevins M, et al.: Int J Periodontics Restorative Dent 2011; 31(4): 367-73.
- McGuire MK & Scheyer ET : J Periodontol 2010; 81(8): 1108-17.
- Cardaropoli D, et al.: J Periodontol 2012; 83(3): 321-28.
- Rotundo R & Pini-Prato G: Int J Periodontics Restorative Dent 2012; 32(4): 413-19.
Does Geistlich Mucograft® need to be treated prior to application to the defect?
Geistlich Mucograft® is ready to be applied directly to the defect and does not need treatment or hydration prior to application. Due to its excellent hydrophilicity, the matrix will hydrate quickly with the patient’s blood after placement in the defect.
Should Geistlich Mucograft® be handled/applied wet or dry?
Geistlich recommends handling and applying the material dry, as it is easy to trim and pre-suture.
Does Geistlich Mucograft® swell after placement?
Our measurements indicate that there is no additional swelling of the matrix following hydration (90-minute period examined)1.
- Data on file, Geistlich Pharma AG, Wolhusen, Switzerland
Can Geistlich Mucograft® be stretched?
No. Geistlich Mucograft® has limited elongation properties and should always be sutured tension-free.
Which side of Geistlich Mucograft® should face the bone?
The compact structure should face outward, away from underlying bone, with the spongeous structure facing the bone or soft-tissue wound bed. No clinical or comparative data is available regarding the performance of Geistlich Mucograft® when placed with the compact structure facing the bone or soft-tissue wound bed.
What suture should be used with Geistlich Mucograft®?
Published data shows different suturing techniques with Geistlich Mucograft®: non-resorbable1,2 and resorbable3, 0-51,2 and 0-64. There is no clinical evidence showing a benefit of one type of suture over another one when suturing Geistlich Mucograft®.
- Sanz M, et al.: J Clin Periodontol 2009; 36(10): 868-76.
- Herford AS, et al.: J Oral Maxillofac Surg 2010; 68(7): 1463-70.
- Cardaropoli D, et al.: J Periodontol 2012; 83(3): 321-28.
- McGuire MK & Scheyer ET : J Periodontol 2010; 81(8): 1108-17.
How many layers of Geistlich Mucograft® should be used?
In the majority of the clinical cases reported and in all the studies performed with Geistlich Mucograft®, one matrix layer was used. Presently, clinical data is not available to prove that several layers of the device outperform a single layer of Geistlich Mucograft®.
Should Geistlich Mucograft® be placed directly over the bone or over the periosteum?
It depends on the indication:
- Gain of keratinized tissue: Geistlich Mucograft® should be applied on a periosteal bed1,2 as direct blood supply is important. In open healing, the blood is supplied through the edges of the device from the surrounding tissue and from the periosteum.
- Recession Coverage: Geistlich Mucograft® can be used partially or completely over bone.3-5 Blood is supplied through the edges of the device from the surrounding tissue and from the flap completely covering the matrix.
- Sanz M, et al.: J Clin Periodontol 2009; 36(10): 868-76.
- Lorenzo R, et al.: Clin Oral Impl Res 2012; 23(3): 316-24.
- McGuire MK & Scheyer ET : J Periodontol 2010; 81(8): 1108-17.
- Cardaropoli D, et al.: J Periodontol 2012; 83(3): 321-28.
- Rotundo R & Pini-Prato G: Int J Periodontics Restorative Dent 2012; 32(4): 413-19.
Should Geistlich Mucograft® be used submerged or in open healing situations?
It depends on the indication:
- Gain of Keratinized Tissue: Open healing is recommended when Geistlich Mucograft® is used to increase the width of keratinized tissue. The innovative design of Geistlich Mucograft® allows excellent healing in open healing situations1.
- Recession Coverage: Geistlich Mucograft® should remain completely submerged under the flap to avoid premature resorption of the collagen.2-4 Direct blood supply is important.
- Sanz M, et al.: J Clin Periodontol 2009; 36(10): 868-76.
- McGuire MK & Scheyer ET: J Periodontol 2010; 81(8): 1108-17.
- Cardaropoli D, et al.: J Periodontol 2012; 83(3): 321-28.
- Rotundo R & Pini-Prato G: Int J Periodontics Restorative Dent 2012; 32(4): 413-19.
Are antibiotics needed after treatment with Geistlich Mucograft®?
After treatment with Geistlich Mucograft®, please follow the same postsurgical management that is usually applied with connective tissue grafts or free gingival grafts.
What is Geistlich Mucograft® Seal?
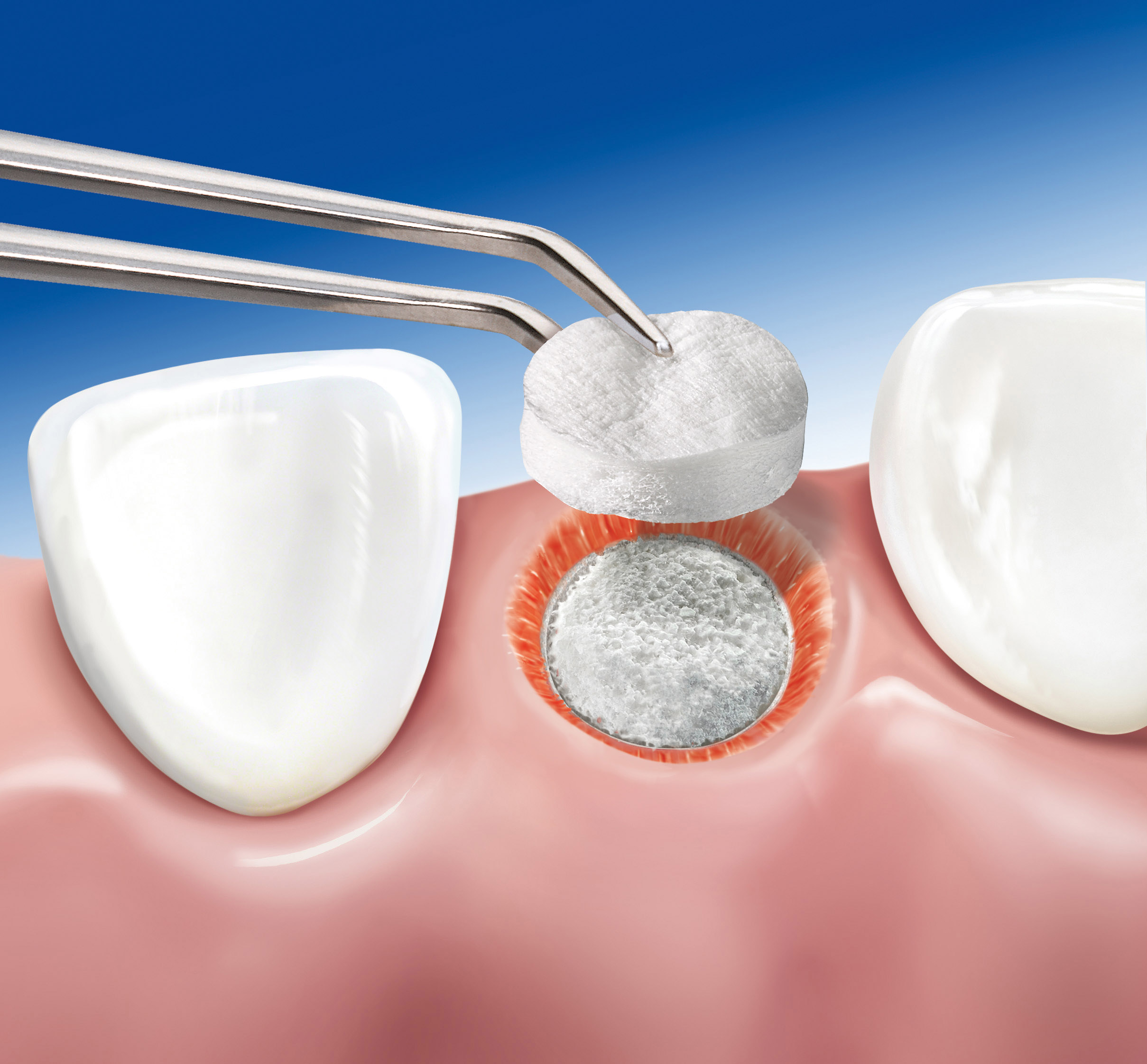
Geistlich Mucograft® Seal is a unique collagen matrix designed specifically for soft tissue regeneration in extraction sockets for ridge preservation
What is Geistlich Mucograft® Seal made of?
Geistlich Mucograft® Seal consists of porcine collagen and is specifically designed for soft tissue regeneration. The matrix is built up of a compact macro-structure that gives stability while allowing open healing, and a spongy micro-structure that supports blood clot stabilization and ingrowth of soft-tissue cells.
Is Geistlich Mucograft® Seal resorbable?
The collagen of Geistlich Mucograft® will be replaced by newly formed soft tissue. The matrix is rapidly vascularized and colonized by soft-tissue cells1,2. This leads to a good integration of the matrix without any signs of foreign body reaction3,4.
- Rocchietta I, et al.: Int J Periodontics Restorative Dent 2012; 32(1): e34-40.
- Ghanaati S, et al.: Biomed Mater 2011 Feb; 6(1): 015010.
- Nevins M, et al.: Int J Periodontics Restorative Dent 2011; 31(4): 367-73.
- Camelo M, et al.: Int J Periodontics Restorative Dent 2012; 32(2): 167-73.
How long is the resorption time for Geistlich Mucograft® Seal?
The resorption time of every biomaterial including Geistlich Mucograft® Seal depends on multiple factors: defect size, metabolism and general health of the patient, among other variables. In order to determine an average resorption time of Geistlich Mucograft® Seal in humans, biopsies of the healing soft tissues would be needed following tooth extraction at different time points. This procedure is not ethically possible to conduct and thus the average resorption time cannot be measured. However, single histologic evaluations indicate that after 8 weeks Geistlich Mucograft® Seal is completely integrated into the newly formed soft-tissue.
Does Geistlich Mucograft® Seal require pre-treatment?
Geistlich Mucograft® Seal is ready to be applied and does not need pre-treatment or hydration before application. Due to its excellent hydrophilicity, the matrix will hydrate quickly following placement into the site with the patient’s blood.
Should Geistlich Mucograft® Seal be handled/applied wet or dry?
Geistlich Biomaterials recommends the handling and application of Geistlich Mucograft® Seal be done in a dry state. The matrix is easy to trim and easy to pre-suture.
Does Geistlich Mucograft® Seal absorb blood and saline equally well?
The hydrophilic properties of Geistlich Mucograft® Seal result in a rapid moistening of the device with either saline or blood.
Does Geistlich Mucograft® Seal expand after placement?
Our measurements indicate that the matrix does not expand further following hydration (90-minute period examined)1.
- Data on file, Geistlich Pharma AG, Wolhusen, Switzerland
Which side of Geistlich Mucograft® Seal should face the bone?
The compact macro-structure should face outward, away from underlying bone, with the spongeous micro-structure facing the bone or soft tissue wound bed. Geistlich Mucograft® Seal spongeous micro-structure is grooved for easier differentiation of the two sides. The grooved spongeous micro-structure should face the bone.
Why should I use Geistlich Bio-Oss Collagen® with Geistlich Mucograft® Seal, if I plan to place an implant after 8-10 weeks?
Geistlich Mucograft® Seal needs the support of Geistlich Bio-Oss Collagen® underneath for good healing of both the soft and the hard tissues in order to preserve the ridge volume. After 8 weeks, the soft-tissues are healed but the mixture of the blood clot, Geistlich Bio-Oss Collagen® and the newly forming bone is still soft. Nevertheless, the implant can be drilled carefully into the socket and the remaining Geistlich Bio-Oss Collagen® will support the volume preservation of the ridge.
Can Geistlich Mucograft® Seal be stretched?
No. Geistlich Mucograft® Seal has limited elongation properties and should always be sutured tension-free.
What suture should be used with Geistlich Mucograft® Seal?
Geistlich Mucograft® Seal should be sutured using non-resorbable sutures; tissue adhesives should not be used1. The close adaptation of the device to the soft tissue borders can be accomplished by single-interrupted, double-interrupted or cross-stitch suturing.
The finest possible suture material comfortably used by the surgeon should be selected: for single-interrupted suturing, a 5.0 or 6.0 suture size is recommended; for cross-stitch suturing, a 5.0 suture size is appropriate1.
- Geistlich Mucograft Seal Advisory Board Meeting Report, 2013. Data on file Geistlich Pharma AG, Wolhusen, Switzerland.
What’s the difference between collagen sponges made by other manufacturers and Geistlich Mucograft® Seal?
Geistlich Mucograft® Seal is specifically designed for soft tissue regeneration1. The collagen of Geistlich Mucograft® Seal is specially processed to support immediate blood clot stabilization. This leads to early vascularization1,3, facilitates soft tissue cell ingrowth2 and excellent integration of the collagen matrix with surrounding tissues2,3. In contrast to other collagen products on the market, Geistlich Mucograft® and Geistlich Mucograft® Seal have been scientifically documented with proven clinical benefits.
- Biocompatibility according to ISO 10993-12001. Data on file Geistlich PharmaAG, Wolhusen, Switzerland.
- Ghanaati S, et al.: Biomed Mater 2011; 6(1): 015010.
- Roccietta I, et al.: Int J Periodontics Restorative Dent 2012; 32(1): e34-40.