Why is a membrane necessary?
Alveolar bone regenerates best with a membrane designed for guided bone regeneration. The use of a membrane leads to increased bone as well as better quality bone compared to procedures that do not include a membrane.1
- Schwarz F, et al.: Clin Oral Impl Res 2008; 19(4): 402-15.
How long should a membrane function as a barrier in Guided Bone Regeneration?
Expert oral surgeons have estimated that a membrane used in Guided Bone Regeneration should maintain its barrier function until the provisional matrix and woven bone are present. As a consequence, barrier duration is considered to be necessary for 4 to 6 weeks, in most cases. Geistlich Bio-Gide® has proven to support bone regeneration on an equivalent level as membranes with a longer barrier function, with the additional benefit of complication-free wound healing.1-4
- Tal H, et al. Clin Oral Impl Res 2008; 19: 295-302.
- Becker J, et al. Clin Oral Impl Res 2009; 20(7): 742-49.
- Schwarz F, et al. Clin Oral Impl Res 2008; 19(4): 402-15.
- Nahles S, et al. Clin Oral Implants Res. 2013 Jul;24(7):812-9.
How does Geistlich Bio-Gide® differ from other collagen membranes?
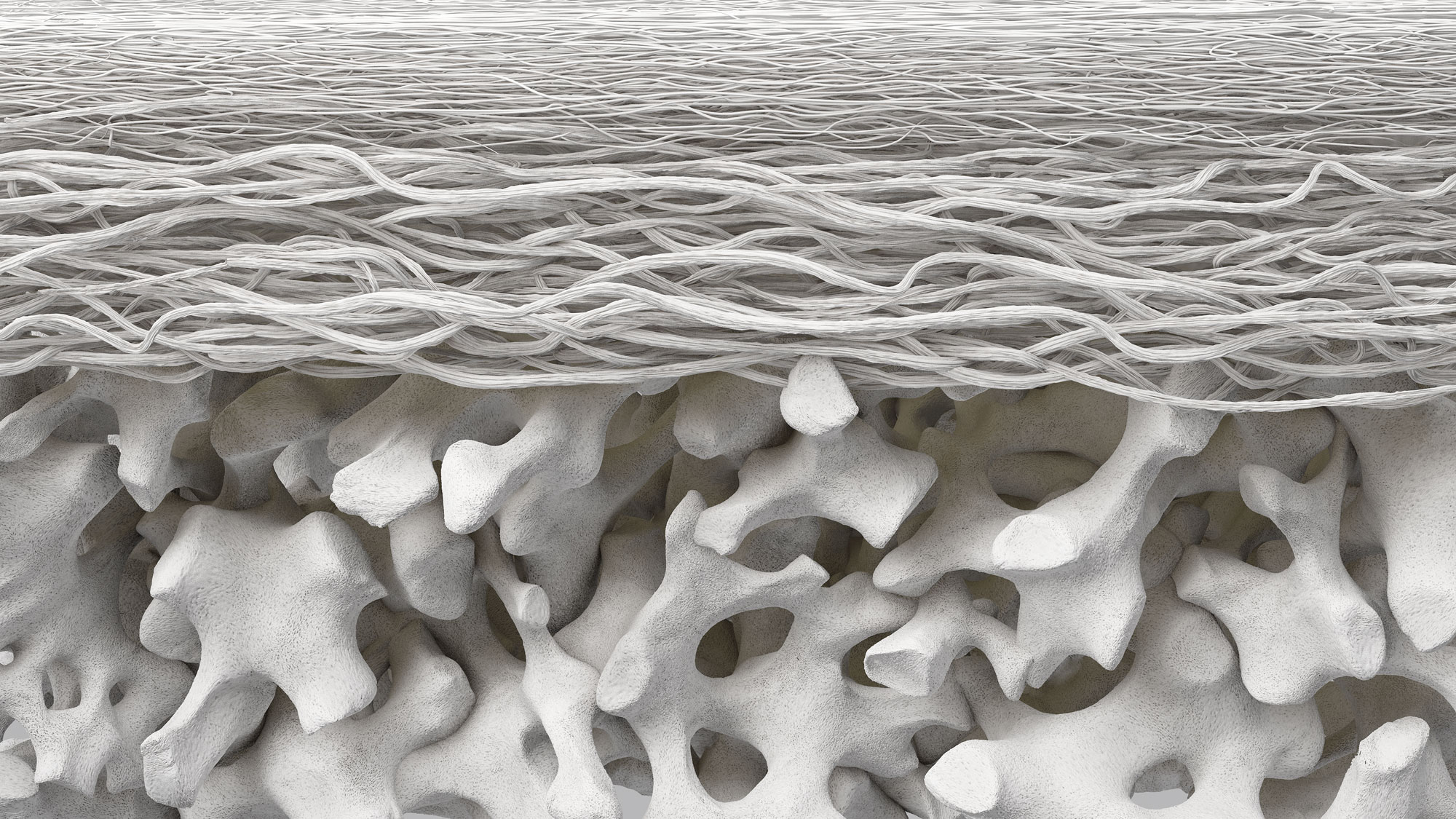
Geistlich Bio-Gide® is a membrane with a unique bilayer structure. It combines optimal bone formation with complication-free wound healing and predictable outcomes. The native collagen of Geistlich Bio-Gide® leads to significantly less soft tissue dehiscences compared to artificially cross-linked membranes.1 A significant number of scientific publications and proven long-term clinical success support the safety and efficacy of Geistlich Bio-Gide®.
- Tal H, et al.: Clin Oral Impl Res 2008; 19: 295-302.
Have inflammatory reactions been associated with Geistlich Bio-Gide®?
Inflammation is a possible complication, which may occur with any surgery. However, biocompatibility of Geistlich Bio-Gide® has been verified in numerous studies.
Is it possible to have an allergic reaction to Geistlich Bio-Gide®?
As Geistlich Bio-Gide® is made of collagen, allergic reactions and inflammatory tissue reactions cannot be excluded. However this is extremely rare.
What happens if the Bio-Gide® membrane is applied with the rough surface towards the soft tissue?
The bilayer structure of Geistlich BioGide® has a compact and porous surface. The compact layer of Geistlich Bio-Gide® exhibits higher cell occlusiveness than the rough, porous layer, preventing the downgrowth of epithelial cells. If the membrane is applied with the porous surface placed toward the soft tissue, integration of the bone cells may take place more slowly. However, it is not necessary to remove the membrane.
Can Geistlich Bio-Gide® be re-sterilized?
No. Autoclaving or hot-air sterilization irreversibly destroys the collagen structure. The physical properties of the membrane are changed and the consistency of the product becomes like parchment paper. In addition the product is not approved for re-sterilization and therefore may not be used.
Does the Geistlich Bio-Gide® membrane have to be fixed?
As Geistlich Bio-Gide® adheres very well to the defect, it is normally not necessary to utilize fixation screws or pins. However, fixation is possible if required.
How can Geistlich Bio-Gide® be used in extraction socket management for open healing?
Geistlich Bio-Gide® can be used submerged or in an open healing situation1depending on the surgeon’s preference. The advantages of the secondary intention healing of Geistlich Bio-Gide® include the ability to perform a flapless surgery and the preservation of the mucogingival line.
- Cardaropoli D, et al.: Int J Periodontics Restorative Dent 2012, 32(4): 421-30.
What is the difference between the two collagen membranes Geistlich Bio-Gide® Compressed and Geistlich Bio-Gide®?
Geistlich Bio-Gide® Compressed has a smoother surface, a firmer feel and is easier to cut compared to Geistlich Bio-Gide®. It offers an alternative handling for clinicians who prefer a firmer membrane.
Does Geistlich Bio-Gide® Compressed need to be stabilized?
As Geistlich Bio-Gide® Compressed adheres very well to the defect, it is normally not necessary to stabilize with screws, pins or sutures. However, stabilization with screws, pins or sutures is possible due to its high tensile strength and may be indicated to avoid displacement due to shear loading or mobilization.
Does the smoother surface of the collagen membrane Geistlich Bio-Gide® Compressed (compared to Geistlich Bio-Gide®) affect wound healing?
The smooth surface of Geistlich Bio-Gide® Compressed does not affect wound healing. It has the same clinical performance and biological properties as Geistlich Bio-Gide®. Various studies have shown that the Geistlich Bio-Gide® family of products supports excellent wound healing1-3 and predictable bone regeneration4.
- Tal H, et al.: Clin. Oral Implants Res 2008; 19: 295-302.
- Zitzmann NU, et al.: Int J Oral Maxillofac Implants 1997; 12: 844-52.
- Becker J, et al.: Clin. Oral Implants Res 2009; 20(7): 742-93.
- Schwarz F, et al.: Clin Oral Implants Res. 2014, 25(9): 1010-15.
What happens if the Geistlich Bio-Gide® Compressed membrane is applied upside down (dense surface facing the bone, porous surface facing the soft-tissue)?
The bilayer structure of Geistlich Bio-Gide® Compressed has a compact, porous surface that exhibits higher cell occlusiveness than the rough, porous layer which prevents the downgrowth of epithelial cells. If the membrane is applied with the porous surface placed toward the soft-tissue, integration of the bone cells may take place more slowly, however the membrane does not need to be removed.
What is the difference between Geistlich Bio-Gide® Shape and Geistlich Bio-Gide®?
Geistlich Bio-Gide® Shape shares the same biological properties but is pre-cut to reduce preparation time, facilitate easier handling and specifically designed for non-intact extraction sockets.
How should Geistlich Bio-Gide® Shape be placed?
Place the membrane without pre-wetting within the extraction socket. The long wing of the Geistlich Bio-Gide® Shape should be placed to cover the bony defect of the socket with the dense surface facing towards the soft-tissue and the rough side facing the defect. To close the extraction socket, both lateral wings of the Geistlich Bio-Gide® Shape membrane should be tucked underneath the mucosa, located mesially and distally of the socket. The upper wing should be tucked lingually/palatally in-between the soft-tissue and bone wall.
Should Geistlich Bio-Gide® Shape be placed inside or outside the alveolus?
The membrane can be placed inside the alveolus for a less invasive surgical procedure but can also be placed outside, between the bone and the soft-tissue. The long wing of the Geistlich Bio-Gide® Shape should cover the bony defect of the socket, if present, with the dense surface facing towards the soft-tissue, and the rough side facing the defect.
Can Geistlich Bio-Gide® Shape be sutured?
Suturing of Geistlich Bio-Gide® Shape on top may be indicated to avoid its displacement due to shear loading or mobilization.
When should Geistlich Bio-Gide® Shape be used versus Geistlich Mucograft® Seal?
Geistlich Bio-Gide® Shape is indicated for the treatment of non-intact extraction sockets. Geistlich Mucograft® Seal is recommended in extraction sockets with intact buccal bone walls.1
- Geistlich Mucograft Seal Advisory Board Meeting Report, 2013. Data on file, Geistlich Pharma AG, Wolhusen, Switzerland.
What is the difference between Geistlich Bio-Gide® Perio and Geistlich Bio-Gide®?
The surface of Geistlich Bio-Gide® Perio has been made firmer when dry to facilitate periodontal applications. Second, the outer blister pack of Geistlich Bio-Gide® Perio includes four sterile water repellent templates. These can be placed repeatedly in the region of the defect to allow customization of the template before trimming the membrane to the precise shape.
Have inflammatory reactions been associated with Geistlich Bio-Gide® Perio?
Inflammation is a possible complication, which may occur with any surgery. However, the biocompatibility of Geistlich Bio-Gide® Perio has been verified in clinical studies and the incidence of complications is low.
Is it possible to have an allergic reaction to Geistlich Bio-Gide® Perio?
As Geistlich Bio-Gide® Perio is made of collagen, allergic and inflammatory tissue reactions cannot be excluded. However, this is extremely rare.
What happens if the Geistlich Bio-Gide® Perio membrane is applied with the rough surface towards the soft tissue?
The bilayer structure of Geistlich Bio-Gide® Perio has a compact and porous surface. The compact layer of Geistlich Bio-Gide® Perio exhibits higher cell occlusiveness than the rough, porous layer, preventing the downgrowth of epithelial cells. If the membrane is applied with the porous surface placed toward the soft tissue, integration of the bone cells may take place more slowly. However, it is not necessary to remove the membrane.
Can Geistlich Bio-Gide® Perio be re-sterilized?
No. Autoclaving or hot-air sterilization irreversibly destroys the collagen structure. The physical properties of the membrane are changed and the consistency of the product becomes like parchment paper. In addition the product is not approved for re-sterilization and therefore may not be used.
Does the Geistlich Bio-Gide® Perio membrane have to be secured with fixation screws or pins?
As Geistlich Bio-Gide® Perio adheres very well to the defect, it is normally not necessary to utilize fixation screws or pins. However, fixation is possible if required.