THE Alternative Soft Tissue Graft

PRODUCT INFORMATION
Ideal Matrix for Volume Stability
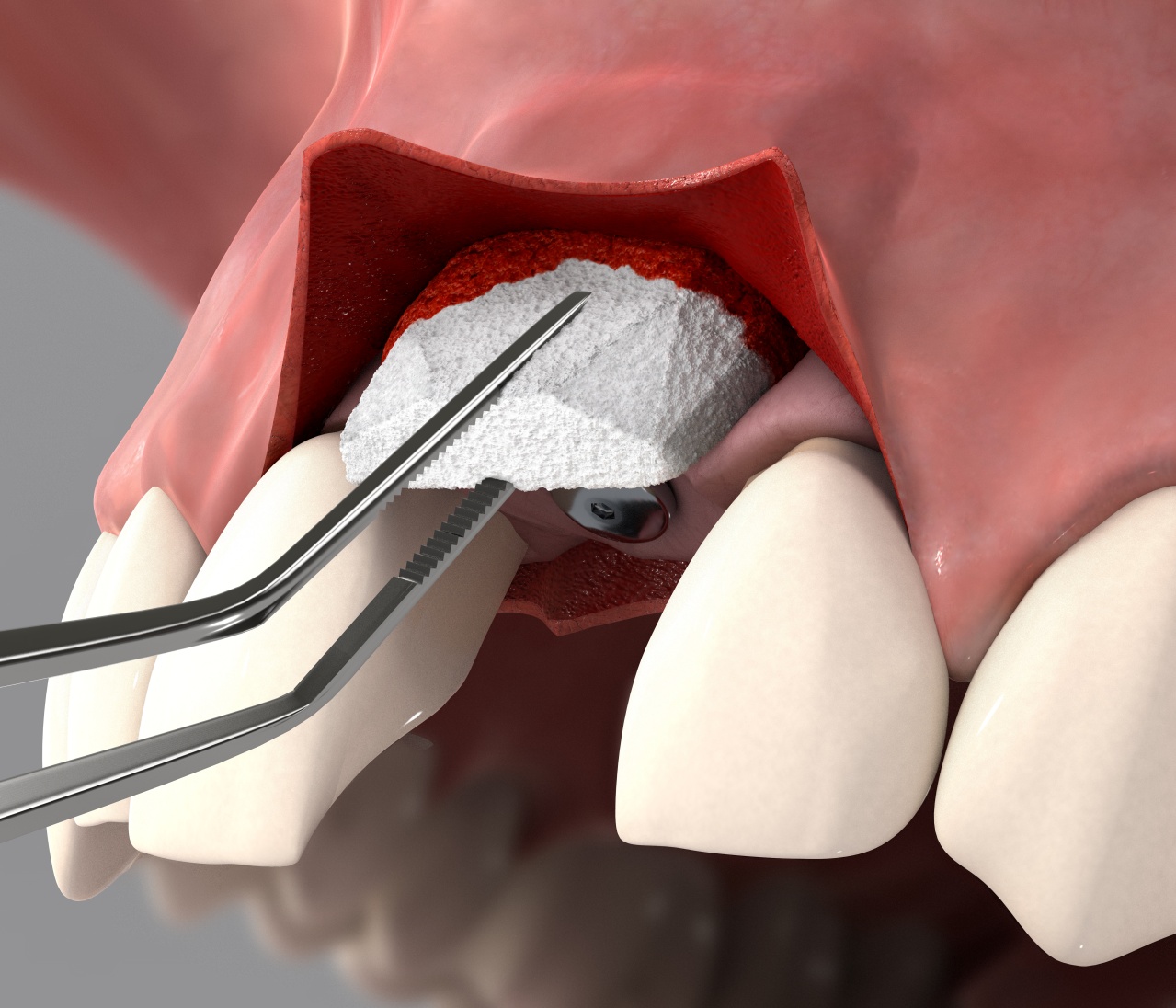
Geistlich Fibro-Gide® is a volume-stable collagen matrix specifically designed for palate free soft tissue regeneration.
- Made of Collagen – a porcine, porous, resorbable, and volume-stable collagen matrix1
- Volume Stability – the reconstituted collagen undergoes smart cross-linking for volume stability of the matrix1
- Supports Soft Tissue Integration – the porous network supports angiogenesis, formation of new connective tissue, and stability of the collagen network in submerged healing situations2,3
- Soft Tissue Formation – animal models have shown good product integration into the surrounding soft tissue while maintaining stability4
UNIQUE BENEFITS
Turning Up the Volume on Patient and Practice Satisfaction
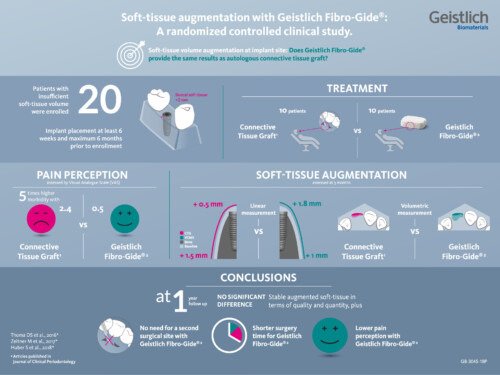
Provides stable augmented soft tissue in terms of both quality and quantity, while eliminating the donor site, shortening surgical time, and lowering patient pain perception.2-3, 5-7
- Fewer Surgical Procedures – Eliminates the need to harvest an autologous graft
- Patient Morbility – No palate required significantly reduces post-operative pain and chair time5, 8-10
- Complication Free Healing – No increase in post-surgical complications were observed with Geistlich Fibro-Gide® vs. a Connective Tissue Graft2
- Patient Preferred – 66.7% of patients prefer Geistlich Fibro-Gide® over connective tissue graft treatment11

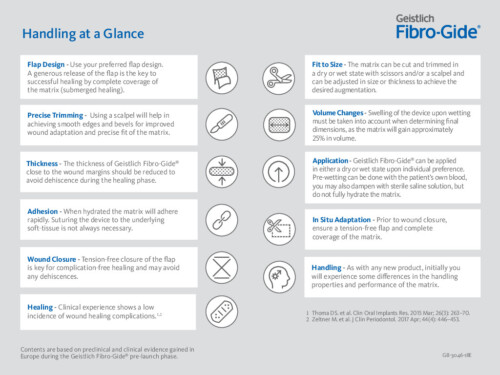
Handling at a Glance
As with any new product, initially, you will experience some differences in the handling properties and performance of the matrix. The instructions below are intended to provide insights for the successful use and application of Geistlich Fibro-Gide®.
Careful Case Selection
When using Geistlich Fibro-Gide®, it is important to carefully consider the patient and desired outcome to determine the appropriate surgical technique.
Flap Design
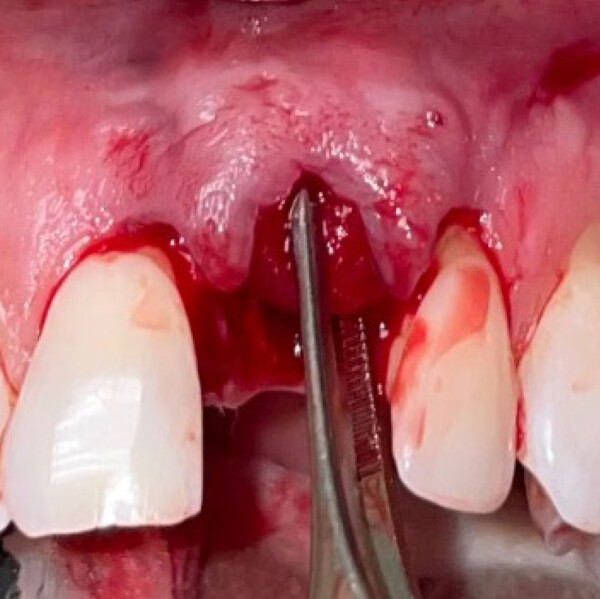
Geistlich Fibro-Gide® can be used in both open flap and flapless procedures. A generous release of the flap is the key to successful healing by complete coverage of the matrix (submerged healing).
Trimming and Cutting
Geistlich Fibro-Gide® can be adjusted in size and thickness, in both a wet or dry state. The use of a scalpel is recommended when the matrix is in a dry state and scissors when in a wet state.
Volume Changes
Swelling of the matrix upon wetting must be taken into account when determining final dimensions, as the matrix will gain approximately 25% in volume.
Thickness
When treating recession defects (Miller Class I/III)*, Geistlich Fibro-Gide® 3 mm thickness is recommended to achieve tension-free wound closure.
*Clinical evidence is continuously being collected for this indication.
Application
Geistlich Fibro-Gide® can be applied in either a dry or wet state upon individual preference. Pre-wetting can be done with the patient’s own blood, you may also dampen with sterile saline solution, but do not fully hydrate the matrix.
Fixation
When hydrated the matrix will adhere rapidly. Suturing the matrix can be performed, however is not always necessary.
Tension-Free Wound Closure
This is key for complication-free healing. It is recommended to bevel the edges of the matrix to accomplish this.
Healing
Primary closure is recommended to ensure maximum soft-tissue thickness gain. In case of exposure Geistlich Fibro-Gide® is forgiving and can heal without additional treatment. Clinical experience shows low incidence of wound healing complications.1,5-8
Post-Operative Instructions
Following the application of Geistlich Fibro-Gide® and during healing, there may be a slight change in the color of the soft-tissue and an increase in volume at the surgical site. Both color matching and a reduction in tissue volume to varying degrees, should be expected over time. It is recommended that this is expressed to the patient, as part of their post-operative instructions, to ensure appropriate expectations, during healing.
Guidance above is based on pre-clinical and clinical evidence gained in Europe and North America during the pre-launch and launch phase of Geistlich Fibro-Gide®.
Handling Geistlich Fibro-Gide®
Geistlich Fibro-Gide®
The Alternative Soft-Tissue Graft
Frequently Asked Questions
Learn about other
Palate Free Solutions
Resources
- Instructions for Use. Geistlich Fibro-Gide®.Geistlich Pharma AG, Wolhusen, Switzerland.
- Thoma DS. et al. J Clin Periodontol. 2016 Oct; 43(10): 874–85 (clinical).
- Thoma DS. et al. Clin Oral Implants Res. 2012 Dec; 23(12): 1333–9 (pre-clinical).
- Thoma DS. et al. Clin Oral Implants Res. 2015 Mar; 26(3): 263–70 (pre-clinical)
- Zeltner M. et al. J Clin Periodontol. 2017 Apr; 44(4): 446–453 (clinical).
- Huber S et al. J Clin Periodontol. 2018 Apr;45(4):504-512 (clinical).
- Chappuis V et al. Int J Periodontics Restorative Dent. 2018 Jul/Aug;38:575-582 (clinical).
- Maiorana C. et al. (2016). Open Dent J. 22(10): 395-410.
- McGuire MK, Scheyer ET. (2016). J Periodontol. 87(3): 221-227.
- Sanz, M. et al. (2009). J Clin Periodontol. 36(10): 868-76.
- McGuire, M. et al. (2022). JPeriodontol. 93(3): 333-342.