Why and how was RPM™ developed?
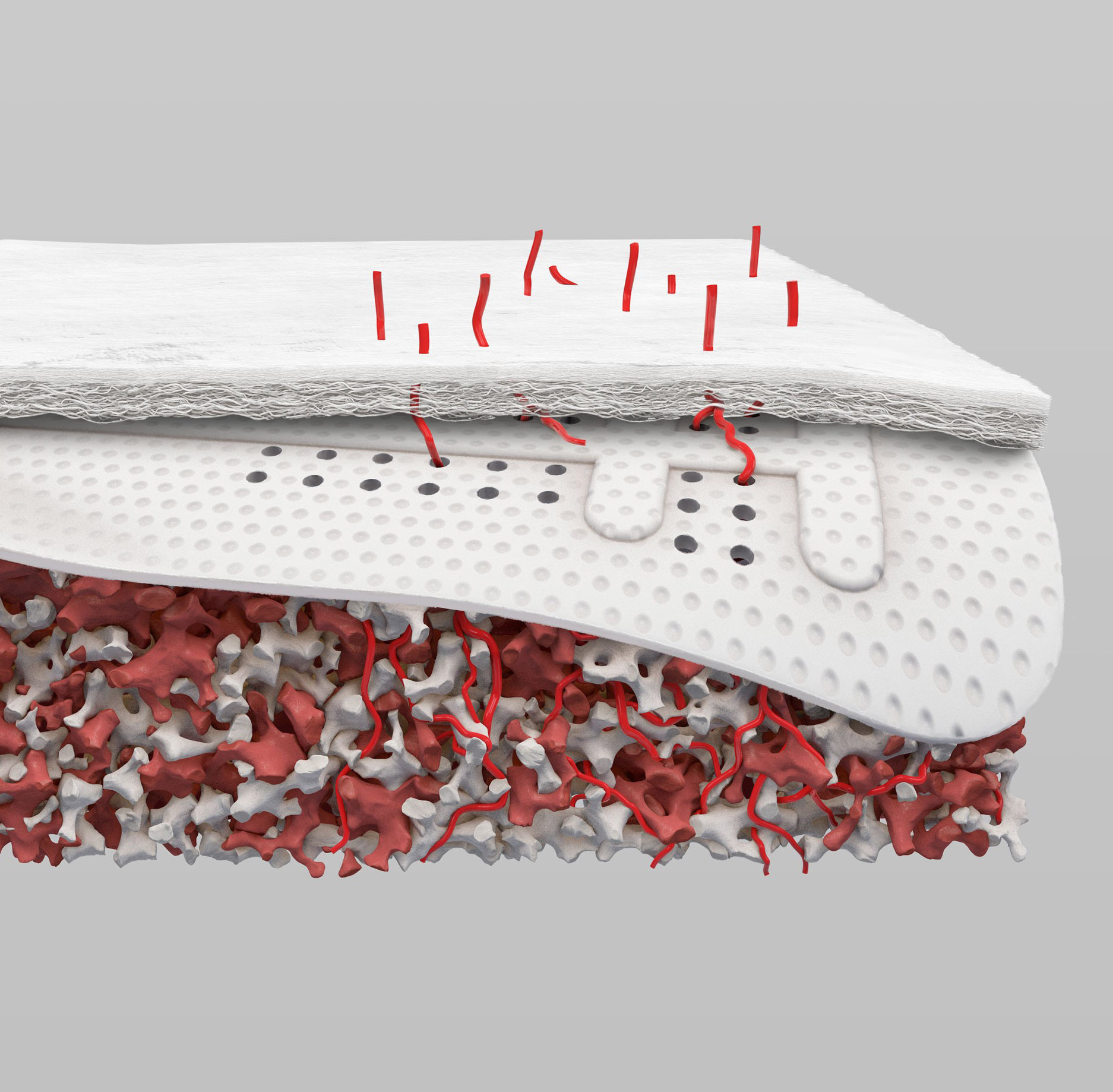
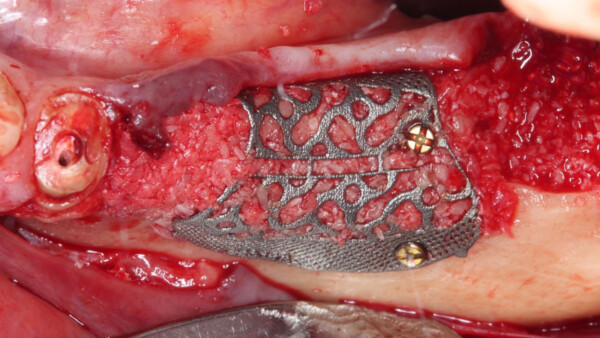
After many years of using both e-PTFE and d-PTFE membranes, Dr. Istvan Urban felt that for larger bone augmentations it was oftentimes challenging to achieve adequate vascularization of the outer sections of the graft due to the distance from the native bone. Dr. Urban began experimenting with perforating d-PTFE membranes, with the notion that the periosteum may be a source of angiogenesis and cell migration that can communicate with the graft. As a result, he was able to achieve clinically more vascularized augmented bone after the healing period.
What type of material is RPM™?
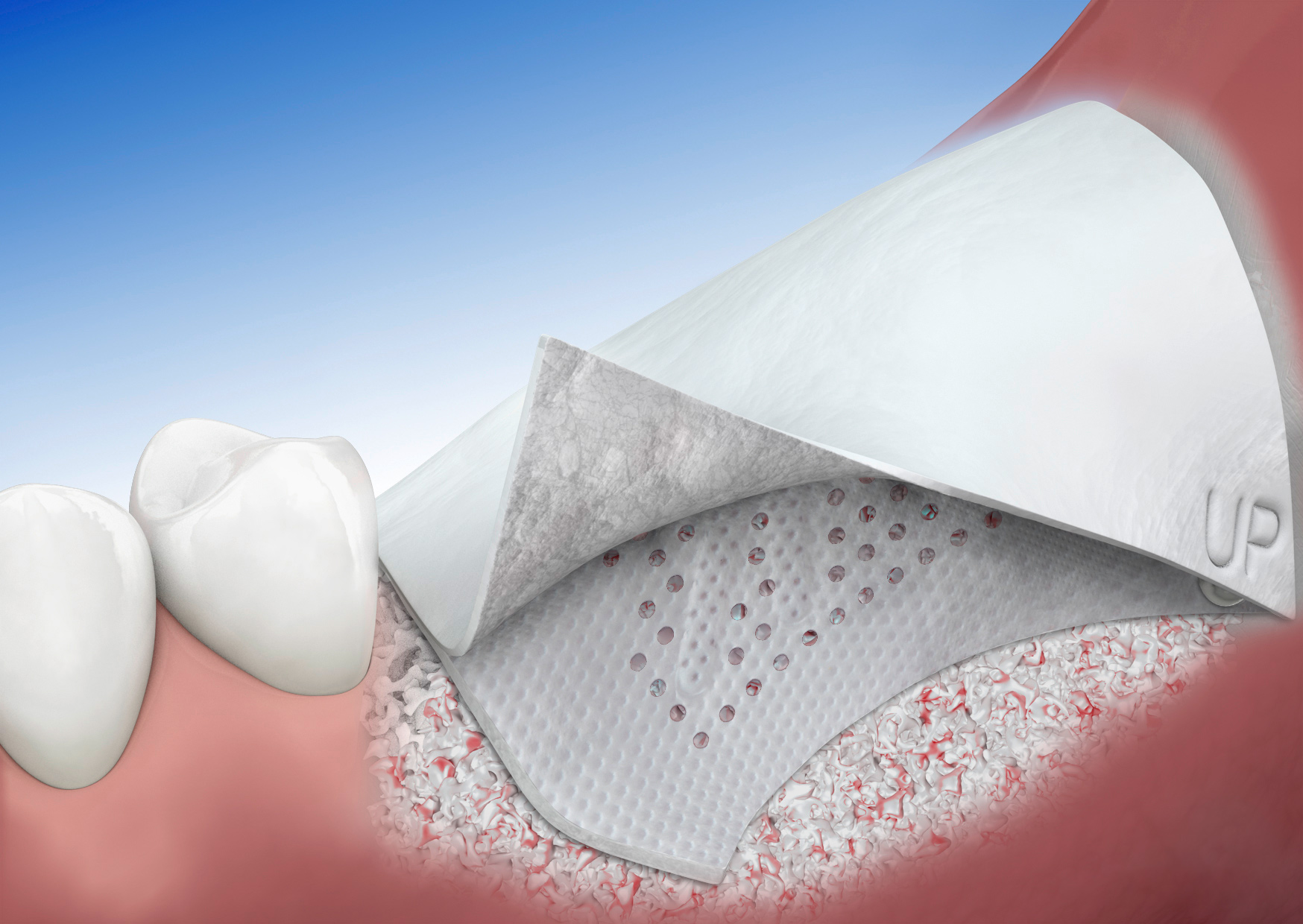
RPM™ is made of two layers of d-PTFE mesh with Grade I titanium. d-PTFE is a dense, biologically inert and biocompatible material, with a grade I titanium frame in between. Grade I titanium is the softest and most ductile grade of commercially pure titanium. It does not have memory and can be easily bent and folded into new shapes.
What is the shelf life of RPM™?
The expiration date of the material is four years from the date of manufacture.
When should I use a regular Ti-reinforced PTFE membrane and when should I use RPM™?
RPM™ and Ti-reinforced PTFE membranes like Cytoplast™ are indicated for the treatment of alveolar bone defects. The rationale behind RPM™ is that the combination of macro-perforations and coverage with Geistlich Bio-Gide®will lead to better vascularization of the outer sections of the augmented bone. According to Osteogenics, Dr. Istvan Urban has been almost exclusively using RPM™ since it has been made available.
Do I have to use Geistlich Bio-Gide® with RPM™?
Although it is possible to use RPM™ alone, Geistlich Bio-Gide® provides additional protection of the graft from potential bacterial or soft tissue infiltration. Geistlich Bio-Gide® also increases tissue tolerance by providing a “buffer” for the soft tissue from the potentially sharp folds and edges of the RPM™. Geistlich Bio-Gide® enhances soft tissue cell migration and early re-epithelialization, which may minimize tissue dehiscence and improve healing. The additional layer of collagen membrane may also provide coverage for graft particles that are not completely protected and stabilized by RPM™
PTFE membranes have a long-standing history as barrier membranes for volume stabilization and are highly biocompatible1. Collagen membranes, such as Geistlich Bio-Gide®, have been shown to be beneficial for uneventful soft tissue healing2, 3 in GBR procedures resulting in protection against soft tissue ingrowth and lowered complication rates2.
- Urban et al. Int J Periodontics Restorative Dent 2013
- Becker et al. Clin Oral Implants Res. 2009
- Tal et al. Clin Oral Implants Res. 2008
If I use Geistlich Bio-Gide® on top of RPM™, do I still need to achieve tension-free primary closure?
Yes. Tension-free primary closure is still crucial to clinical success when performing ridge augmentation procedures using RPM™ and Geistlich Bio-Gide®. Because of the macro-perforations of the RPM™, early tissue dehiscence may lead to bacterial infection through the mesh. Even though Geistlich Bio-Gide® provides additional barrier coverage, it can resorb prematurely in the event of exposure and not offer protection from bacteria.
How long should the bone graft heal before re-entry to place implants?
Generally, for large augmentations (>6mm) it is recommended for the clinician to wait at least 9 months for the bone to mature enough prior to implant placement.
How should I fixate RPM™?
Either a screw or pin/tacking system could be used to fixate RPM™. Fixation through the titanium frame and/or the areas of macro perforations should be avoided. Geistlich Bio-Gide® does not need to be fixated over RPM™.
Can RPM™ only be used for vertical ridge augmentation?
Even though RPM was designed for challenging GBR procedures like vertical ridge augmentation, it can certainly be used for horizontal augmentation, as well.
It has been shown that Geistlich Bio-Gide® is perfectly suited for horizontal GBR (i.e. Sausage Technique), however there are clinicians that prefer a more form-stable barrier for large augmentations. RPM™ in combination with Geistlich Bio-Gide® provides a perfect solution to that.
How do I manage exposure?
Exposure without purulence – Weekly monitoring and cleaning with chlorhexidine gel and gentle brushing. If exposure occurs before 2 months post-operatively, the mesh should stay in place 6-8 weeks before removal. If exposure occurs after 2 months post-operatively, monitor until full graft maturation if possible1
Exposure with purulence – Antibiotics can be prescribed at the practitioner’s discretion and the mesh and all non-incorporated graft particles and granulomatous tissue should be removed. If exposure occurs before 2 months post-operatively, all of the graft may be compromised and require removal. If exposure occurs after 2 months post-operatively, there may be some consolidated regenerated tissue underneath the infected or granulomatous areas1
Please note that the above guidelines describe the management of exposed conventional titanium-reinforced D-PTFE membranes. Exposed RPM™ may be more prone to infection due to the presence of macro perforations.
- Gallo et al. J Oral Maxillofacial Implants 2019
Should I trim RPM™ before use? How do I trim the material?
Some customization and adjustment to the size/shape of the material is often necessary to ensure placement without contacting adjacent teeth or other anatomical structures.
Trimming can be done with surgical scissors. Care should be taken during cutting to avoid delamination of the material. Cutting through the areas of macro perforations or titanium frame may weaken the material.
Should I over-augment the graft in anticipation of graft resorption?
Obtaining tension-free primary closure is crucial to the healing process and success of the graft. Over-augmentation can make flap management and primary closure difficult. The clinician should use sound judgment to ensure that an adequate amount of graft is applied without significantly interfering with the clinician’s ability to close the tissue.
Is there any published clinical data on RPM™?
Since RPM™ was just launched in 2019, there is limited published research at this time. Dr. Istvan Urban has been using the product exclusively since its launch and has published an article in Clinical Oral Implants Research.1
- Clin Oral Implants Res, 2021 Jul;32(7):828-839 doi: 10.1111/clr.13755. Epub 2021 Apr 24
What is the uniqueness of RPM™ over PTFE membranes?
The unique circular perforations of RPM™ allow for direct contact between the bone graft and periosteum, favoring naturally occurring vascularization and infiltration of osteogenic cells into the bone graft.
What is the purpose of the mandibular SKUs?
The mandible SKUs were designed with perforations only on the buccal side of the mesh. This allows perfusion of blood and osteogenic cells to the buccal aspects of the graft. The non-perforated lingual side allows easier removal from the lingual aspect, as there is no tissue infiltration.
I am currently using titanium meshes, what is the benefit of RPM™?
Have you ever seen non-vital or non-incorporated bone graft when you remove the membrane due to lack of vascularity in the outer sections of the bone graft? The perforations of RPM allow dual vascularization (from bone and periosteum) to increase the vitality of the bone graft.