THE PROBLEM
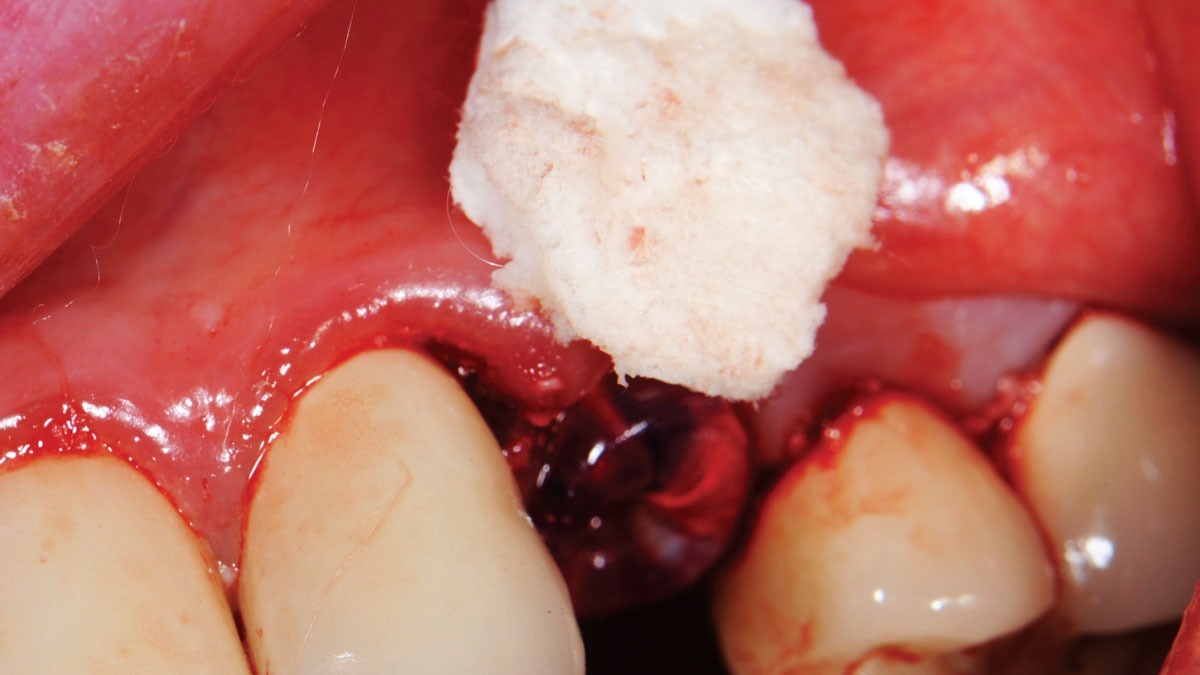
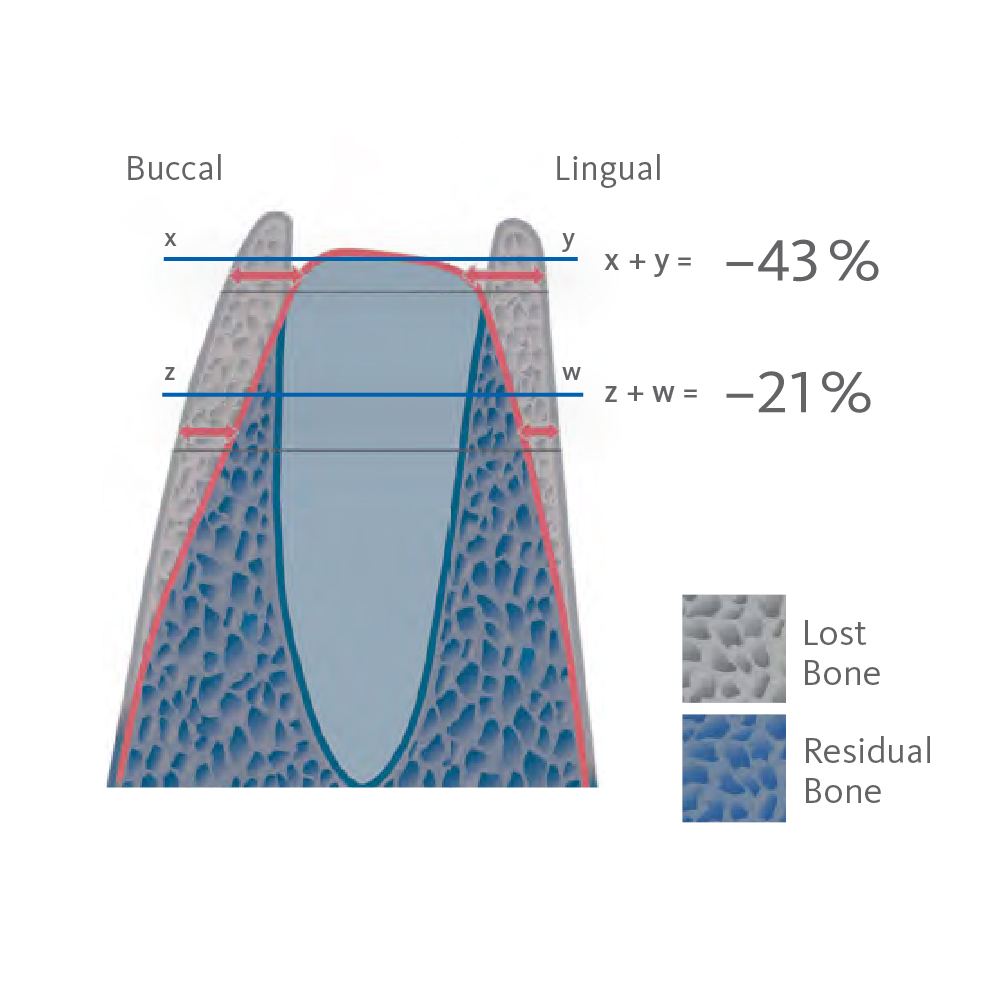
Without Ridge Preservation, the bone starts to resorb, leading to a loss of volume and density.
This can complicate future implant procedures, requiring additional bone and soft tissue grafting surgeries. This, in turn, increases treatment time and cost and can potentially lead to suboptimal treatment outcomes.1-6
Spontaneous healing causes around 50% of the alveolar volume to be lost within six months (horizontal 29–63%; vertical 11–22%).7-9 The use of collagen plugs is not suitable to preserve bone10. Without supportive bone, the buccal and labial soft tissue in the socket collapses. As a result, the natural formation of new bone in the socket cannot compensate for the loss of alveolar volume.11
The Benefits of Ridge Preservation:
User-Friendly & Time Saving
The use of Geistlich biomaterials in ridge preservation procedures offers a simplified approach. Resulting in less pain and discomfort for your patient during healing.18
Two treatment solutions tailored to your needs:
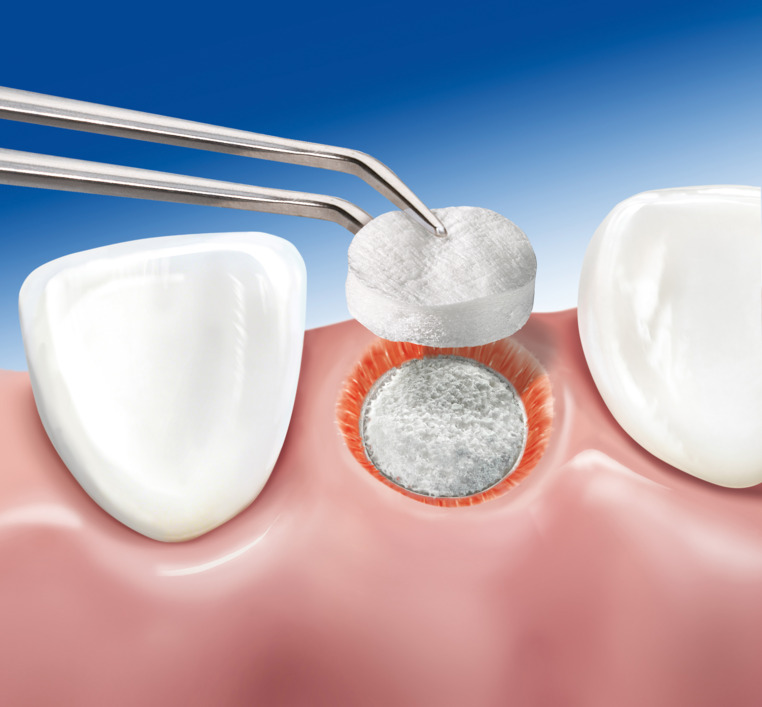
SCENARIO 1
Ridge Preservation with an intact extraction socket
This includes Geistlich Bio-Oss Collagen® to fill the extraction socket with a long-term, volume-stable bone substitute, and Geistlich Mucograft® Seal to replace the soft tissue component and to increase the tissue thickness and long-term stability of peri-implant bone margins.
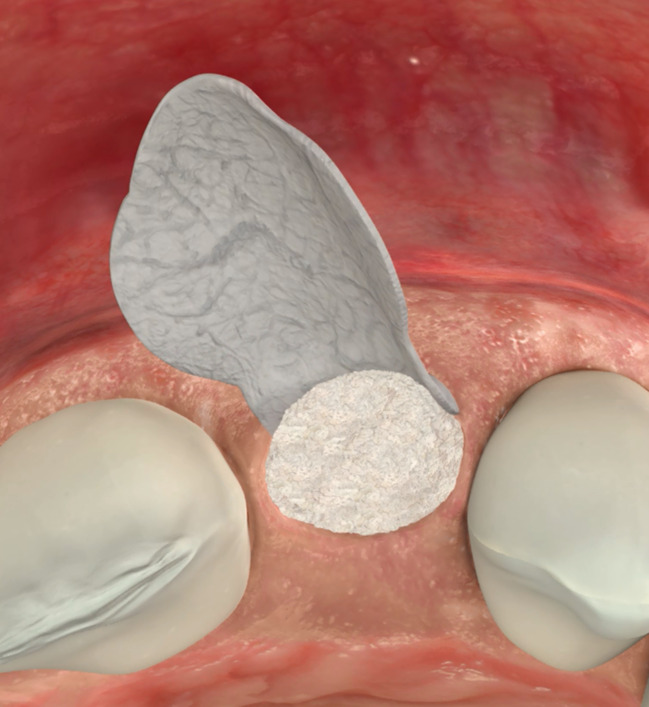
SCENARIO 2
Ridge Preservation with a non-intact extraction socket
This includes Geistlich Bio-Oss Collagen® to fill the extraction socket with a long-term, volume-stable bone substitute, and Geistlich Bio-Gide® Shape to cover the bone graft from the buccal and occlusal sides, preventing the ingrowth of fibroblasts, maximizing the volume of bone preserved.
When faster turnover of bone is desired,
vallos® allografts are ideal.
For delayed implant placement after ridge preservation:
vallos® Mineralized Cortical Allograft
For early implant placement after ridge preservation:
vallos® Mineralized Cancellous Allograft
Related Content

Alveolar Ridge Preservation with vallos® Mineralized Cortico-Cancellous Allograft
Ridge Augmentation and Delayed Implant Placement on an Upper Lateral Incisor
Horizontal Ridge Augmentation in the Esthetic Zone
Knowledge Boost:
Predictability and Esthetic Outcomes in Ridge Preservation Cases
Geistlich Bio-Oss® and Geistlich Bio-Gide® product families have undergone rigorous research in 44 clinical studies on Ridge Preservation.29
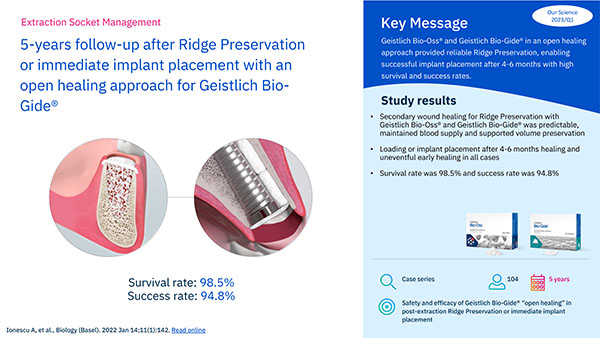
5-years follow-up after Ridge Preservation or immediate implant placement…

Mixing Allograft and Xenograft for a Predictable Alveolar Ridge Preservation Procedure: A Case Series
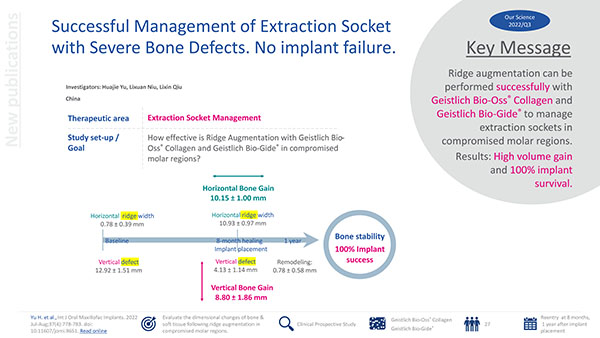
Successful Management of Extraction Socket with Severe Bone Defects. No implant failure
What can I do to simplify my ridge preservation
procedures and save time?
Geistlich offers a comprehensive portfolio of biomaterials for delayed implant placement and ridge augmentation, ensuring successful patient outcomes, ease of use and time savings.


With 90% Geistlich Bio-Oss® granules and 10% porcine collagen Geistlich Bio-Oss Collagen® provides long-term volume stability due to its low resorption rate, which helps preserve the ridge volume.19-21


Geistlich Bio-Gide® Shape is a ready to use pre-trimmed resorbable collagen membrane that reduces preparation time. It has been developed specifically for non-intact extraction sockets and is easy to apply due to a slower uptake of moisture which allows for exact positioning of the material. 6,22-25
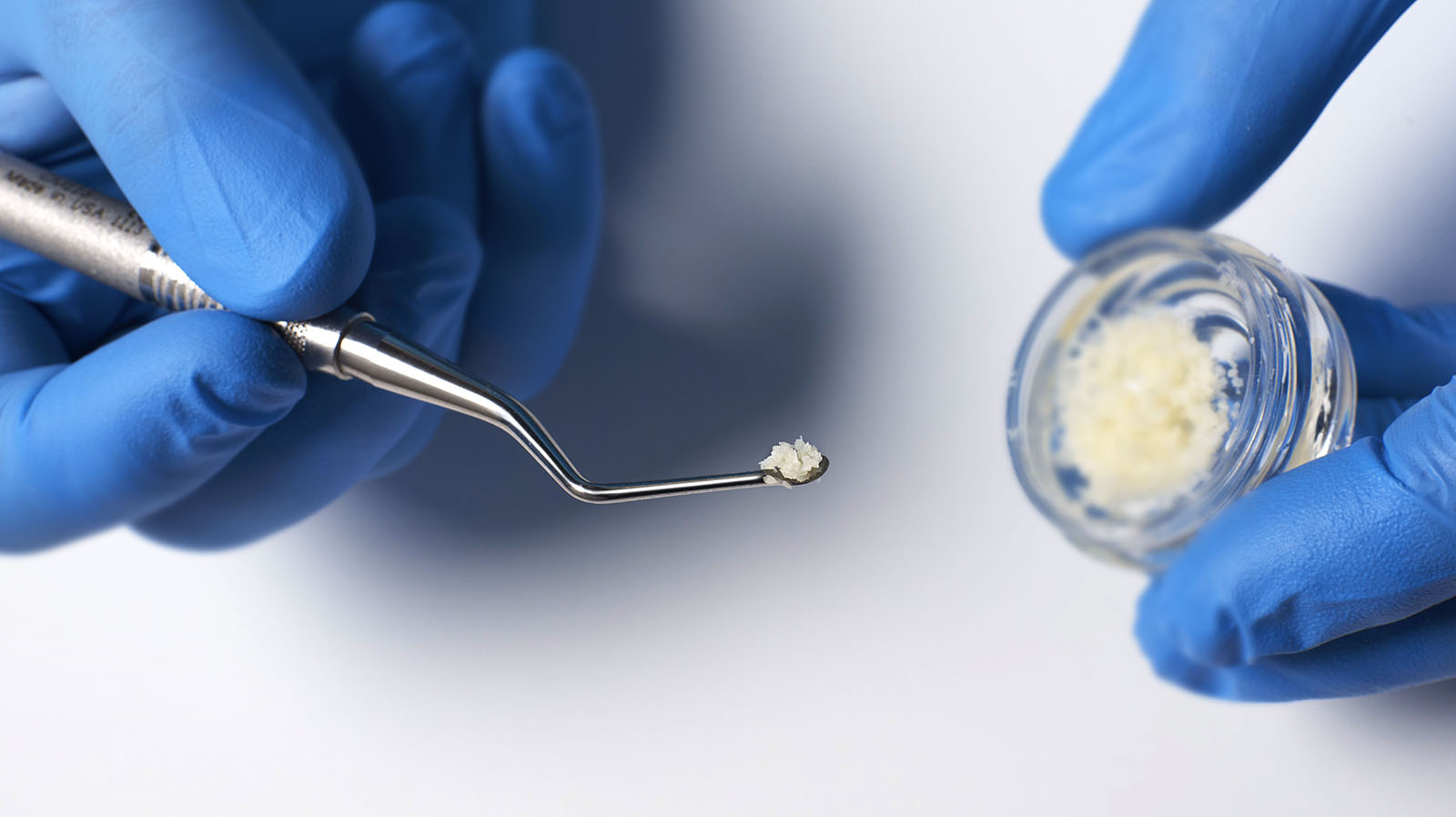

vallos® allografts are natural bone graft substitutes, which due to the preservation of inherent biological properties, provides the optimal characteristics and scientific properties required for bone formation.27
- Schropp L, et al.: Int J Periodontics Restorative Dent. 2003; 23(4): 313-23.
- Van der Weijden F, et al.: J Clin Periodontol. 2009; 36(12): 1048-58.
- Sanz M, et al.: Clin Oral Implants Res. 2010; 21(1): 13-21.
- Hammerle CH, et al.: Clin Oral Implants Res. 2012; 23 Suppl. 5: 80-82.
- Jung RE, et al.: J Clin Periodontol. 2013; 40(1): 90-98.
- Weng D, et al.: Eur J Oral Implantol. 2011; 4 Suppl: 59-66.
- Perelman-Karmon et al.: Int J Periodontics Restorative Dent. 2012; 32(4):459-65.
- Tan WL, et al.: Clin Oral Implants Res. 2012; 23 Suppl: 5:1-21.
- Cardaropoli D, et al.: Int J Periodontics Restorative Dent. 2012; 32(4):421-30.
- Hämmerle C, et al.: Clin Oral Implants Res. 2012; 23 Suppl. 5: 80–82.
- Araújo M. Clin Oral Implants Res. 2014; 12. doi: 10.1111/clr.12366.
- Lim HC, et al.: J Clin Periodontol. 2019; 46(11): 1144-54.
- Cardaropoli D, et al.: Int J Periodontics Restorative Dent. 2014; 34(2): 211-7.
- Jonker BP, et al.: Clin Oral Impl Res. 2021; 32: 123–33.
- Cardaropoli D, et al.: Int J Periodontics Restorative Dent. 2015; 35(5): 677-85.
- Geistlich Mucograft® Seal Advisory Board Meeting Report, 2013. Data on file, Geistlich Pharma AG, Wolhusen, Switzerland.
- Schlee M, Esposito M: Eur J Oral Implantol. 2009; 2(3):209-17.
- Ionescu A et al.: Biology (Basel). 2022; 11(1): 142.
- Araújo MG, et al.: Clin Oral Implants Res. 2010; 21(1):55-64.
- Mordenfeld A, et al.: Clin. Oral Implant Res. 2010;21(9):961–70.
- Junker R, & Behring J, J Osseointegr. 2023; 15(2):113-123.
- Morjaria, KR. et al.: Clin Implant Dent Relat Res. 2014; 16(1): 1-20.
- Horvath, A. et al.: Clin Oral Investig. 2013; 17(2): 341-63.
- Vittorini Orgeas, G. et al.: Int J Oral Maxillofac Implants. 2013; 28(4): 1049-61.
- Vignoletti, F. et al.: Clin Oral Implants Res. 2012; 23Suppl. (5): 22-38.
- Malorana, C. et al.: Open Dent J. 2016; 22(10): 395-410.
- Data on file, MTF Biologics
- GEM 21S® Instructions for Use.
- Hu et al.: Oral Surgery. 2022;15:163–169.