Simplified Solutions for Long-Term Success in Complex Cases
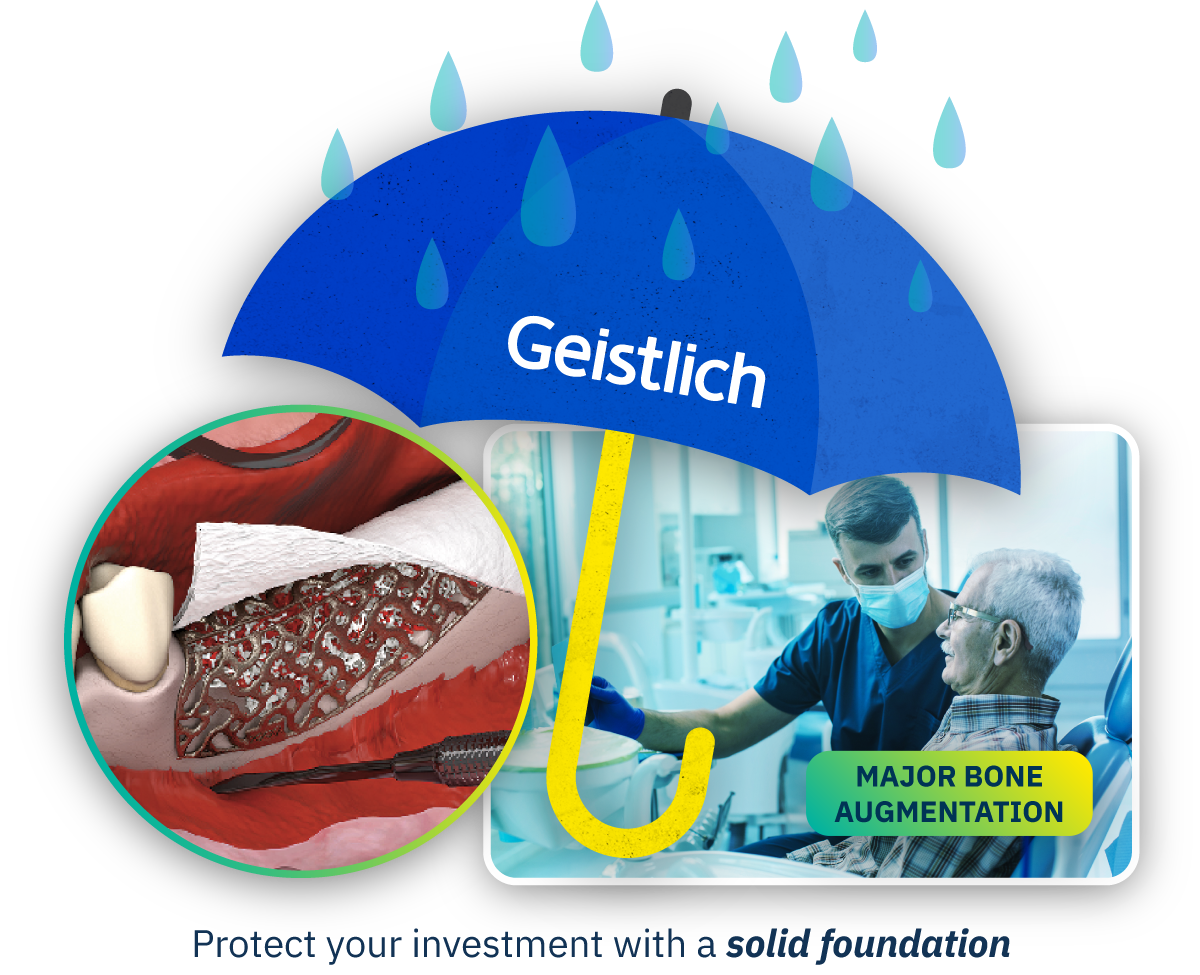
Challenges of Traditional Solutions for Major Bone Augmentation
Traditionally, creating a stable and regenerative environment in large bony defects can be invasive, time consuming and unpredictable, leading to:
Time consuming surgery and slow healing time




Maintaining volume1


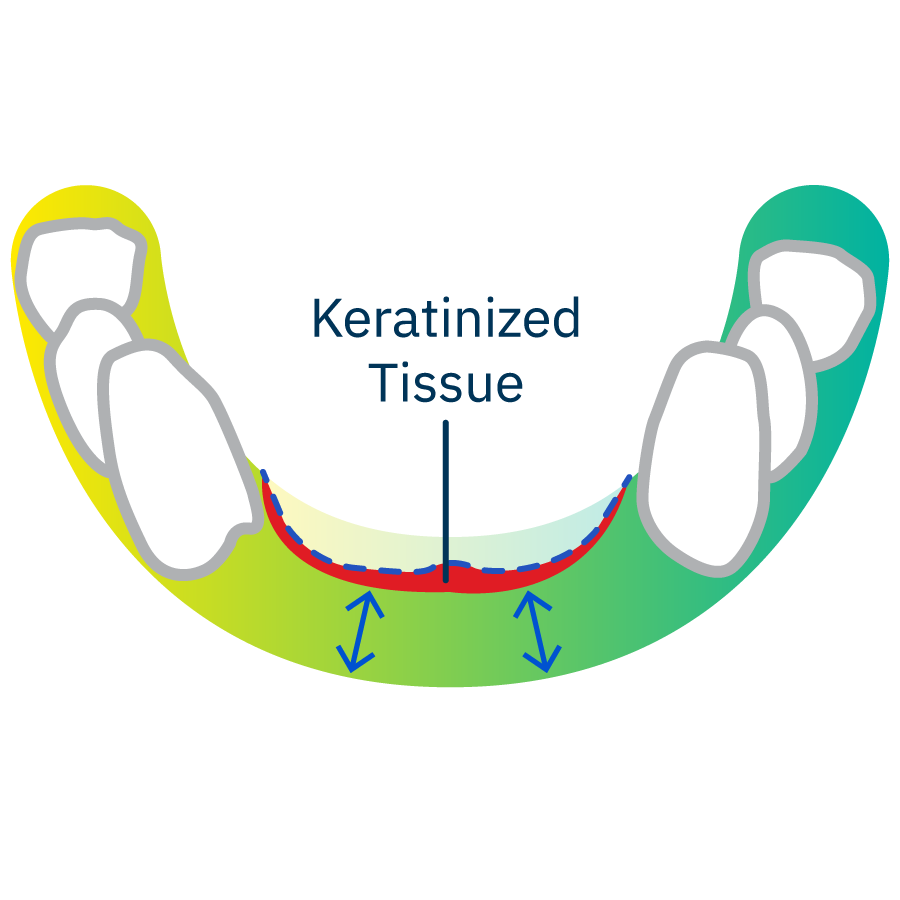
Insufficient vestibular depth and keratinized tissue or tissue thickness after wound closure



Patient morbidity after harvesting autogenous tissue




Insufficient alveolar ridge width and height for implant placement


More Bone. More Time
Simplified Solutions to Oral Surgery Excellence
The combination of the best-in-class biomaterials and 3-D printing technology offers you more bone in complex bone augmentation and opportunity to reduce surgery time without complex adaptations.2







Select a
Product
to Learn
More
Yxoss CBR®
The first customized 3D-printed bone regeneration solution for complex bone defects. The customized precision fit allows surgeons to save on time-consuming cutting, shaping, or adapting of titanium mesh.
New Innovations Coming Soon, Yxoss Fully Protect®
Geistlich Bio-Oss®
Thanks to its high resorption stability and osteoconductivity, Geistlich Bio-Oss® protects human bone grafts against degradation.1, 18-20
vallos®
vallos® allografts are natural bone graft substitutes, which due to the preservation of inherent biological properties, provides the optimal characteristics and scientific properties required for bone formation.3
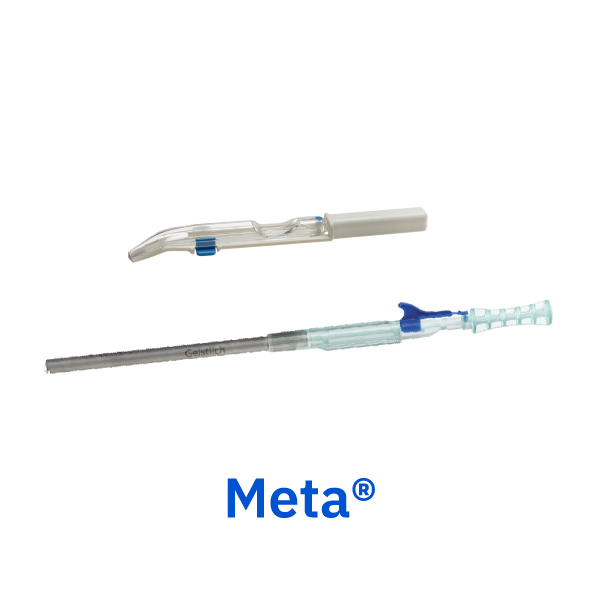
Meta®
Geistlich bone harvesting instruments provide an easy method for harvesting autogenous cortical bone for use in grafted sites with a minimally invasive technique.
GEM 21S®
A growth-factor enhanced matrix promotes faster, better healing, new bone formation, and more predictable outcomes.4
Geistlich Bio-Gide®
The native collagen membrane provides a barrier function long enough to protect the newly forming bone from soft-tissue ingrowth and provides support for wound healing.1, 5-11
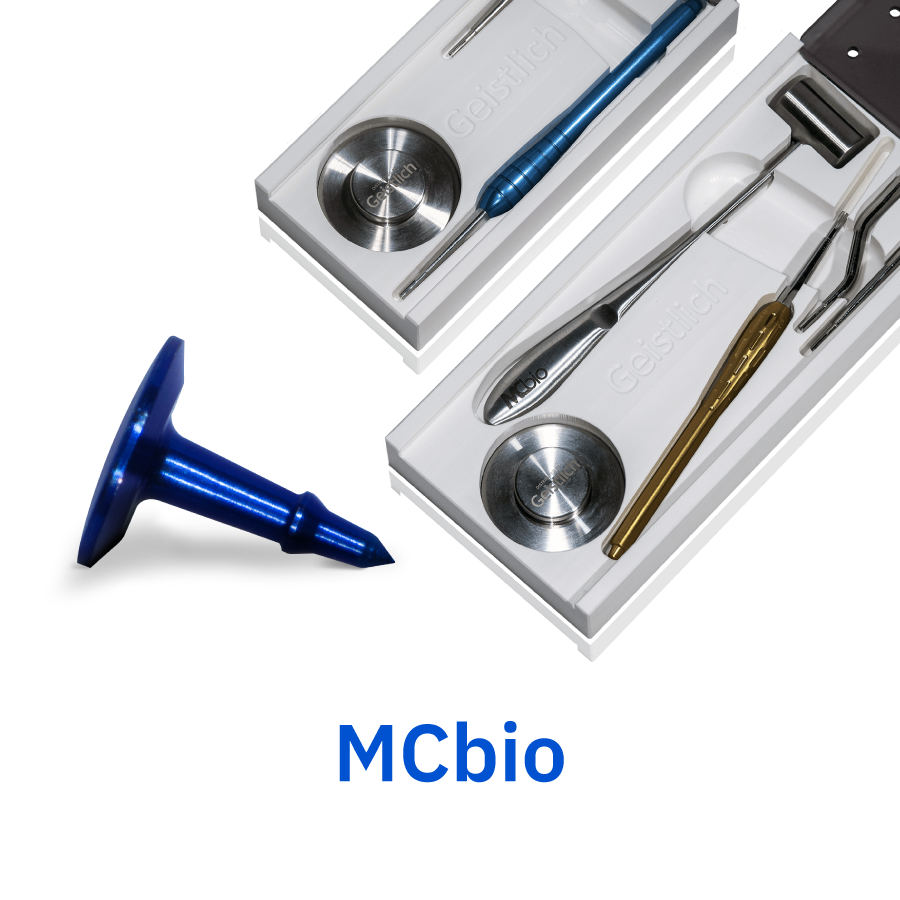
McBio
Our Supertack requires no pre-drilling of the site and can be used in very compact bone. The unique tack shape allows for easy removal and blue tack coloring facilitates detection under soft tissues.
Geistlich Fibro-Gide®
Geistlich Fibro-Gide® offers the ideal matrix for volume stability while significantly reducing post-operative pain and chair time.12-14
Geistlich Mucograft®
Geistlich Mucograft® is a unique collagen matrix designed specifically for the gain of keratinized tissue and for recession coverage.14,16,17 18,19 Like Geistlich Fibro-Gide, the palate free innovation eliminates the need to harvest an autologous graft, reduces patient morbidity, and faster healing.15
Fill out the form to access the webinar.
What are the documented clinical advantages of using Geistlich Products?
Application of Geistlich biomaterials in conjunction with innovative treatment solutions can offer customized, reinforced and well-documented clinical outcomes.
- Improved patient satisfaction20-28
- Optimal application and handling
- Reduced surgery time12-14
- Less invasive surgery15
- Fewer complications9, 36, 39
- Lower morbidity40
- Stable clinical outcome29-33
- Less bone resorption29-33
- Long-term implant survival rate 22,26,27,37
- Predictable bone gain 22, 26, 27
- Improved healing with Geistlich Bio-Gide® 28,29, 34-36
- Rapid healing and bone formation with GEM 21S®4
Overall, more bone volume and improved bone quality lead to higher implant survival rates38
Related Content
Mandibular Alveolar Ridge Split with Delayed Implant Placement
Horizontal Ridge Augmentation in the Posterior Mandible of a 90-Year-Old Female

Combined Horizontal and Vertical Regeneration using a CAD-CAM Titanium Scaffold
Knowledge Boost:
Predictability and Esthetic Outcomes in Major Bone Augmentation Cases
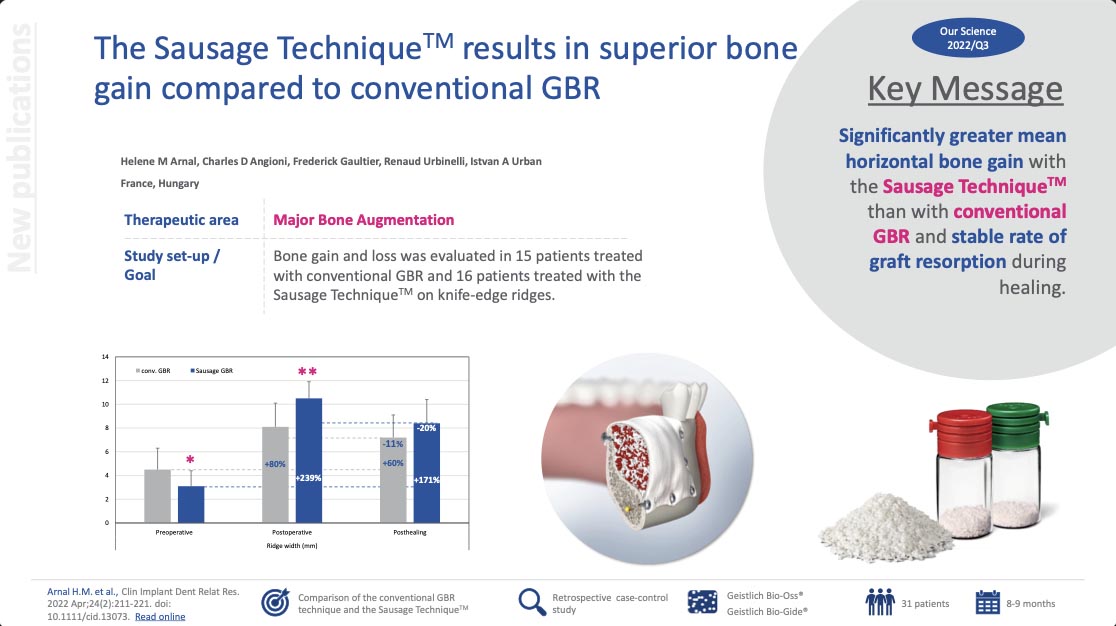
The Sausage TechniqueTM results in superior bone gain compared to conventional GBR
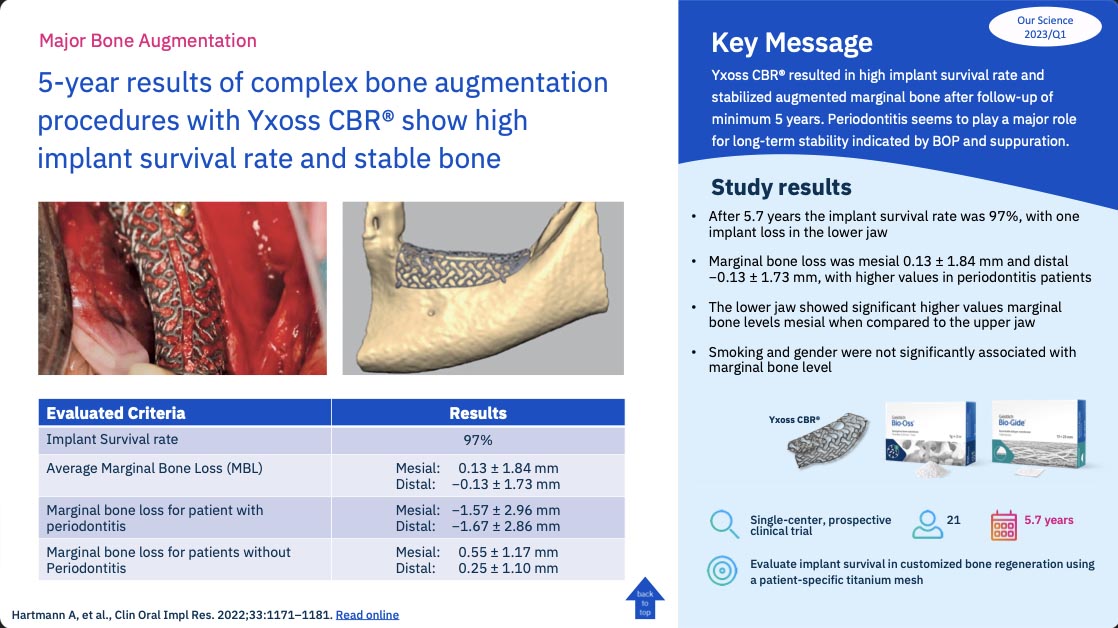
5-year results of complex bone augmentation procedures with Yxoss CBR® show high implant survival rate and stable bone
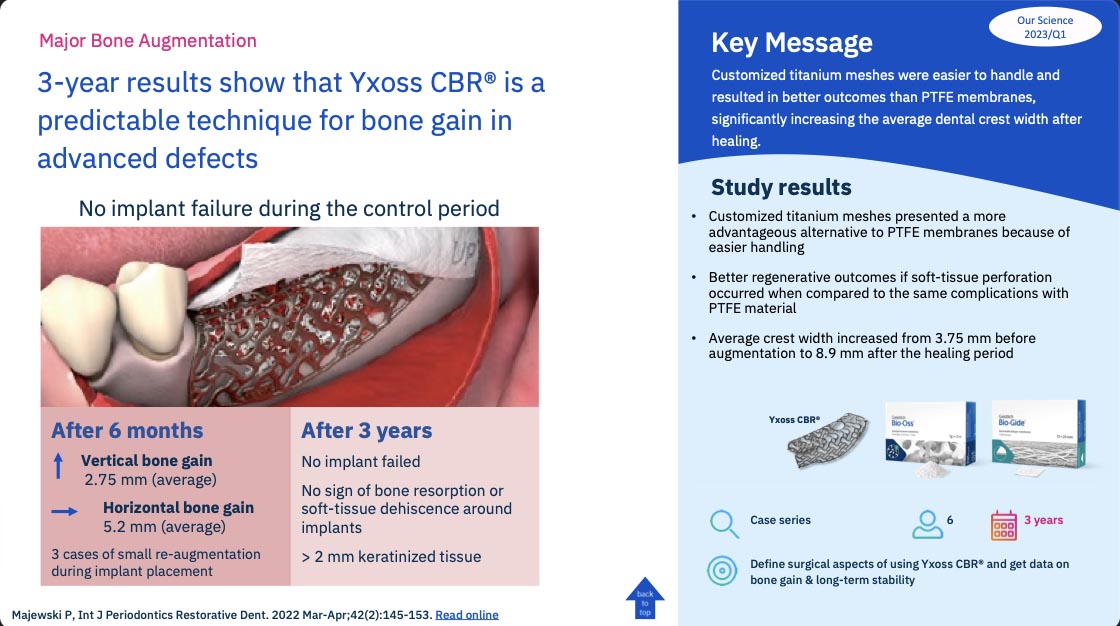
3-year results show that Yxoss CBR® is a predictable technique for bone gain in advanced defects
- Schlegel KA et al.: Int J Oral Maxillofac Implants. 2003 Jan–Feb;18(1):53–8.
- Sagheb, K. et al. (2017). Int J Implant Dent. 3(1), 36.
- Data on file, MTF Biologics
- GEM 21S® Instructions for Use.
- Schwarz F et al.: Clin Oral Implants Res. 2014 Sep;25(9):1010–5. (Clinical study)
- Orsini G et al.: Oral Dis. 2007 Nov;13(6):586–93. (Clinical study)
- Mordenfeld A et al.: Clin Oral Implants Res. 2010 Sep;21(9):961–70. (Clinical study)
- Lindgren C et al.: Int J Oral Maxillofac Implants. 2009 Nov-Dec;24(6):1093–100.(Clinical study)
- Becker J et al.: Clin. Oral Implants Res. 2009 Jul;20(7):742–9. (Clinical study)
- Rothamel D et al.: Clin Oral Implants Res. 2005 Jun;16(3):69–378. (Pre-clinical study)
- Rothamel D et al.: Int J Oral Maxillofac Implants. 2012 Jan–Feb;27(1):146–54.(Pre-clinical study)
- Zeltner M. et al. J Clin Periodontol. 2017 Apr; 44(4): 446–453 (clinical).
- Maiorana C. et al. (2016). Open Dent J. 22(10): 395-410.
- Sanz, M. et al. (2009). J Clin Periodontol. 36(10): 868-76.
- Thoma D, et al. (2012). J Clin Periodontol. 39(2): 157-65.
- Konter U, et al.: Deutsche Zahnärztliche Zeitschrift 2010; 65: 723-30.
- Lorenzo R, et al.: Clin Oral Impl Res 2012; 23(3): 316-24.
- McGuire MK & Scheyer ET: J Periodontol 2010; 81(8): 1108-17.
- Cardaropoli D, et al.: J Periodontol 2012; 83(3): 321-28.
- Li et al., 2013 Implant Dentistry 22(2):p 112-116, April 2013.
- Felice et al., 2009 Clin Oral Implants Re. 2009 Dec;20(12):1386-93.
- Urban et al., (2013). Int. J Periodontics Restorative Dent.2013 May-Jun;33(3):299-307.
- Merli et al., 2013 Eur J Oral Implantol. 2013 Spring;6(1):27-37.
- De Santis et al., 2012 Journal of Craniofacial Surgery 23(3):p e186-e189, May 2012.
- Trevisiol L et al. J Carniofac Surg 2012, 23(5): 1343-48.
- Chiapasco et al., 2013 Minerva Stomatol 2013; 62 (1-2),3-16.
- Jung, R et al. (2013). Clin Oral Implants Res. 24(10):1065-73.
- Schwarz, F. et al. (2008). Clin Oral Implants Res. 19(4): 402-409
- Von Arx, T. et al. (2006). Clin Oral Implants Res. 17(4): 359-356.
- Canullo et al., 2006 Int J Periodontics Restorative Dent. 2006 Aug;26(4):355-61.
- Maiorana, C et al. Int J Periodontics Restorative Dent. 2005 Feb;25(1):19-25.
- Maiorana, C et al. Open Dent J. 2011 April 25;5:71-78
- Cordaro et al., 2011 Clin Oral Implants Res. 2011 Oct;22(10):1145-1150.doi: 10.1111
- Kim et al., 2008 In Vivo. 2008 Mar-Apr;22(2):231-6
- Reddy KP, Nayak DG, Uppoor A AS. J Contemp Dent Pract 2006 February;(7)1:060-070.
- Tal H, et al.: Clin. Oral Implants Res 2008; 19: 295-302.
- Aghaloo T. Moy PK. Int J Oral Maxillofac Implants. 2007:22 Suppl:49-70.
- Pjetursson, BE. et al. (2008). J Clin Periodontal. 35: 216-240.
- Zitzmann NU, et al.: Int J Oral Maxillofac Implants 1997; 12: 844-52.
- Chiapasco M. et al Clin Oral Implants Res. 2021 Apr;32(4):498-510


