What are the indications for Geistlich Fibro-Gide®?
Geistlich Fibro-Gide® is intended for soft-tissue augmentation. It is indicated for localized gingival augmentation to increase keratinized tissue (KT) around teeth and implants, alveolar ridge reconstruction for prosthetic treatment, and recession defects for root coverage.1
- Instructions for Use. Geistlich Fibro-Gide®. Geistlich Pharma AG, Wolhusen, Switzerland.
What is Geistlich Fibro-Gide® made of?
Geistlich Fibro-Gide® is a porous, resorbable and volume-stable porcine collagen matrix specifically designed for soft tissue regeneration.1 The matrix is made of reconstituted collagen and undergoes smart chemical cross-linking to improve volume stability of the device while maintaining its excellent biocompatibility.1-3
- Instructions for Use. Geistlich Fibro-Gide®. Geistlich Pharma AG, Wolhusen, Switzerland.
- Thoma DS et al. J Clin Periodontol. 2016 Oct; 43(10): 874–85.
- Thoma DS et al. Clin Oral Implants Res. 2015 Mar; 26(3): 263–70.
Is Geistlich Fibro-Gide® resorbable?
Animal models show gradual resorption and integration of Geistlich Fibro-Gide® into host tissue while maintaining volume stability.1
- Thoma DS. et al. Clin Oral Implants Res. 2012.
Is Geistlich Fibro-Gide® an alternative to free gingival graft (FGG) or to connective tissue grafts (CTG)?
Geistlich Fibro-Gide® is an alternative to autologous connective tissue grafts (CTG) for soft tissue volume augmentation around dental implants and natural teeth.1,2Geistlich Fibro-Gide® obviously has the advantage of reducing patient morbidity since there is no additional harvest site. Furthermore, there is minimal risk of necrosis when using Geistlich Fibro-Gide® compared to CTG.3
- Thoma DS et al. J Clin Periodontol 2016.
- Zeltner M et al. J Clin Periodontol 2017.
- Data on file. Geistlich Pharma AG, Wolhusen, Switzerland.
Does Geistlich Fibro-Gide® need pre-treatment?
Geistlich Fibro-Gide® is ready to be applied in a dry or wet state to the defect and does not need pretreatment or conditioning before application. Geistlich Fibro-Gide®possesses excellent hydrophilic characteristics that lead to a rapid hydration of the collagen matrix (patient’s own blood and/or sterile saline).1 When applied dry to the defect site, the matrix will hydrate easily by soaking up the patient’s own blood and/or sterile saline if applied to the matrix, very quickly.1
- Data on file. Geistlich Pharma AG, Wolhusen, Switzerland.
Is it easier to apply/handle Geistlich Fibro-Gide® in a dry or wet state?
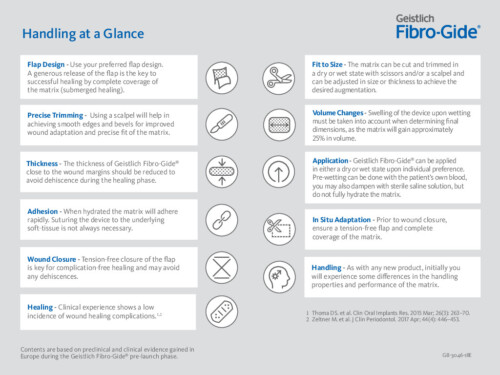
Geistlich Fibro-Gide® can be applied either in a dry or wet state subject to individual preference: pre-wetting can be done with blood and/or sterile saline.1,2 Geistlich Fibro-Gide® can be cut and trimmed both in a dry or wet state, using scissors and/or a scalpel.1,2 The scalpel is recommended for dry handling.1,2 Using a scalpel will help to obtain smooth edges and tapers for improved wound adaptation and precise fit of the matrix.1,2
- Instructions for Use. Geistlich Pharma AG, Wolhusen, Switzerland.
- Data on file. Geistlich Pharma AG, Wolhusen, Switzerland.
Does Geistlich Fibro-Gide® increase its volume after hydration?
The device will gain approximately 25% in volume once wet. This increase in volume has to be taken into account when deciding to trim the matrix and determining its dimension prior to implatation.1 A generous flap design is the key to full submersion of the matrix.
- Data on file. Geistlich Pharma AG, Wolhusen, Switzerland.
Which flap design is recommended when using Geistlich Fibro-Gide®?
Use your preferred flap design. A generous preparation of the flap and ensuring adequate blood supply is key to promoting successful healing by complete coverage of Geistlich Fibro-Gide® (submerged healing). Different surgical techniques, such as tunneling and/or envelope techniques are currently being clinically tested.
What suture technique should be used with Geistlich Fibro-Gide®?
Clinical experience has shown that tension-free wound closure is a key factor in preventing dehiscence. In open flap procedures for augmentation at the alveolar ridge a horizontal mattress suture is recommended to stabilize the matrix (5.0-6.0).1 In recession coverage, single sutures (7.0) have been used for stabilization.1
- Data on file. Geistlich Pharma AG, Wolhusen, Switzerland.
How should the flap be closed with Geistlich Fibro-Gide®underneath?
A tension-free closure of the flap is key to a successful and complication-free healing and avoidance of any dehiscences during the healing phase.
Are antibiotics needed after treatment with Geistlich Fibro-Gide®?
After surgical treatment with Geistlich Fibro-Gide®, post-surgical management that typically follows procedures involving connective tissue grafts has been reported to be effective. Please use your clinical judgment based on the individual profile of the patient and the details of the surgery.
What is the difference between Geistlich Mucograft® and Geistlich Fibro-Gide®?
Geistlich Mucograft® = Compact and spongy layer
Geistlich Fibro-Gide® = Porous layer
Geistlich Mucograft® = Reconstituted collagen: No cross-linking
Geistlich Fibro-Gide® = Reconstituted collagen: Smart cross-linking
Geistlich Mucograft® = Reduced volume stability
Geistlich Fibro-Gide® = Good volume stability
Geistlich Mucograft® = Open-healing & submerged healing
Geistlich Fibro-Gide® = Submerged healing only
Geistlich Mucograft® Treatment Option:
- Gain of keratinized tissue
- Socket Seal
- Vestibuloplasty
- Recession Coverage
Geistlich Fibro-Gide® Treatment Option:
- Soft tissue volume augmentation around dental implants and natural teeth, and under pontics
- Recession Coverage
How much thickness of Geistlich Fibro-Gide® is required in order to obtain a thickening of the soft-tissue comparable to when connective tissue graft (CTG) is used?
Clinical studies have shown a physiological increase in thickness when using CTG as the gold standard of 0.35 to 3.2 mm.1-3 Clinical studies have shown that treatment with Geistlich Fibro-Gide® results in an increase of 1-2 mm of thickness4,5 which is equivalent to results obtained with CTG4. Geistlich Fibro-Gide® is provided with a thickness of 6 mm which gives freedom to the surgeon to trim it to the desired size and to use the collagen matrix for thickening of tissues under pontics where more volume might be required. For lateral soft tissue augmentation, it is recommended to reduce the thickness of Geistlich Fibro-Gide® in order to achieve a tension-free, primary wound closure.
- Eghbali A et al. Clin Implant Dent Relat Res. 2016.
- Thoma DS et al. Periodontol 2000. 2014.
- De Bruyckere T et al. J Clin Periodontol. 2015.
- Thoma DS et al. J Clin Periodontol 2016.
- Chappuis V et al. Manuscript in preparation.
How does Geistlich Fibro-Gide® perform if exposed in the oral cavity?
Geistlich Fibro-Gide® was designed for and performs best with primary wound closure. Clinical observation suggests that under circumstances where the wound dehiscence led to limited exposure of Geistlich Fibro-Gide®, uneventful healing and successful outcomes were nonetheless achieved.
How do I access the IFUs for Geistlich products?
The IFUs for Geistlich products can be found using this page.