Bassam M. Kinaia DDS, MS, DICOI
Sterling Heights, Michigan, USA
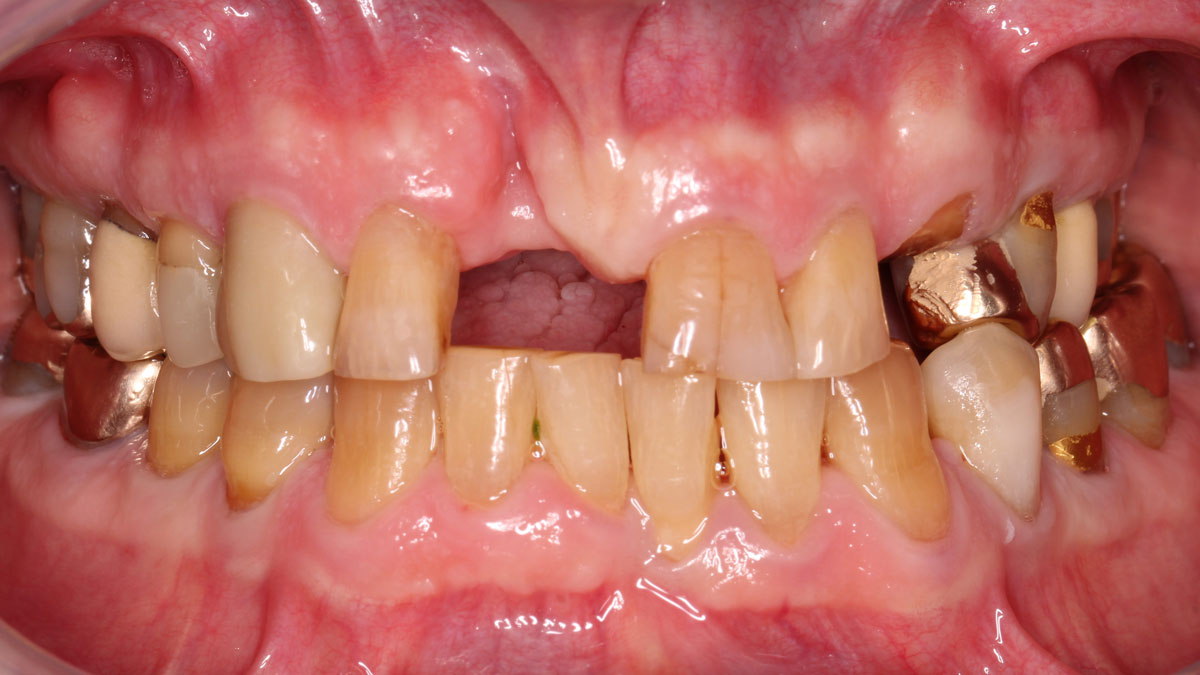
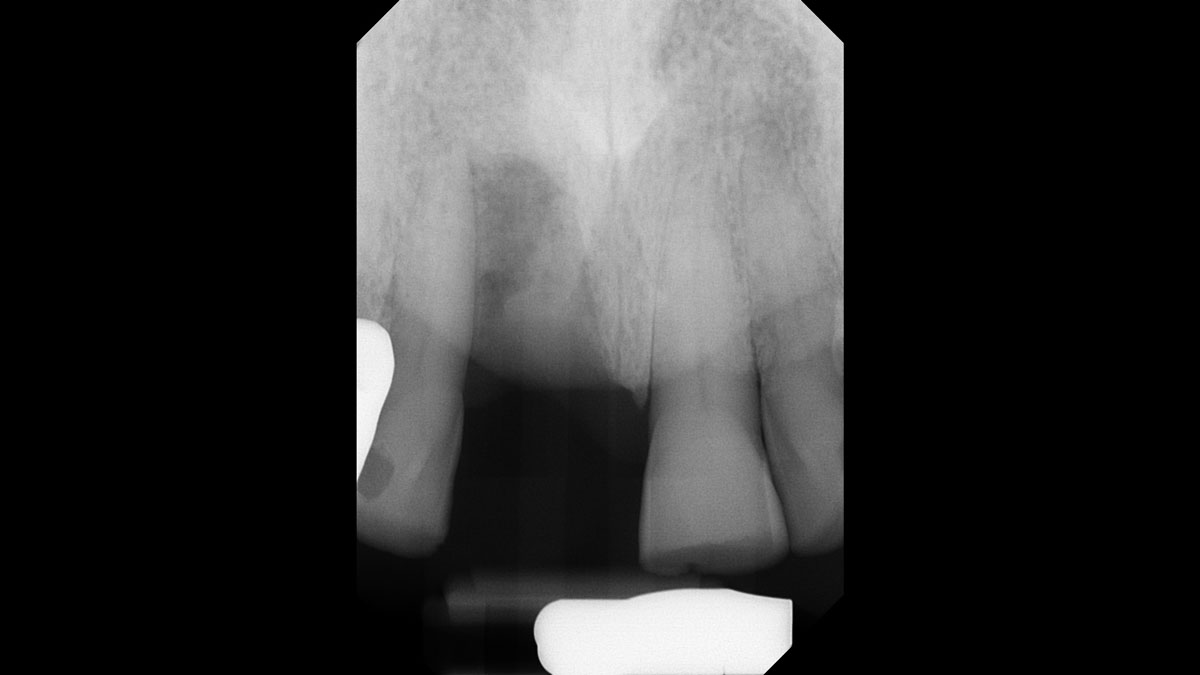
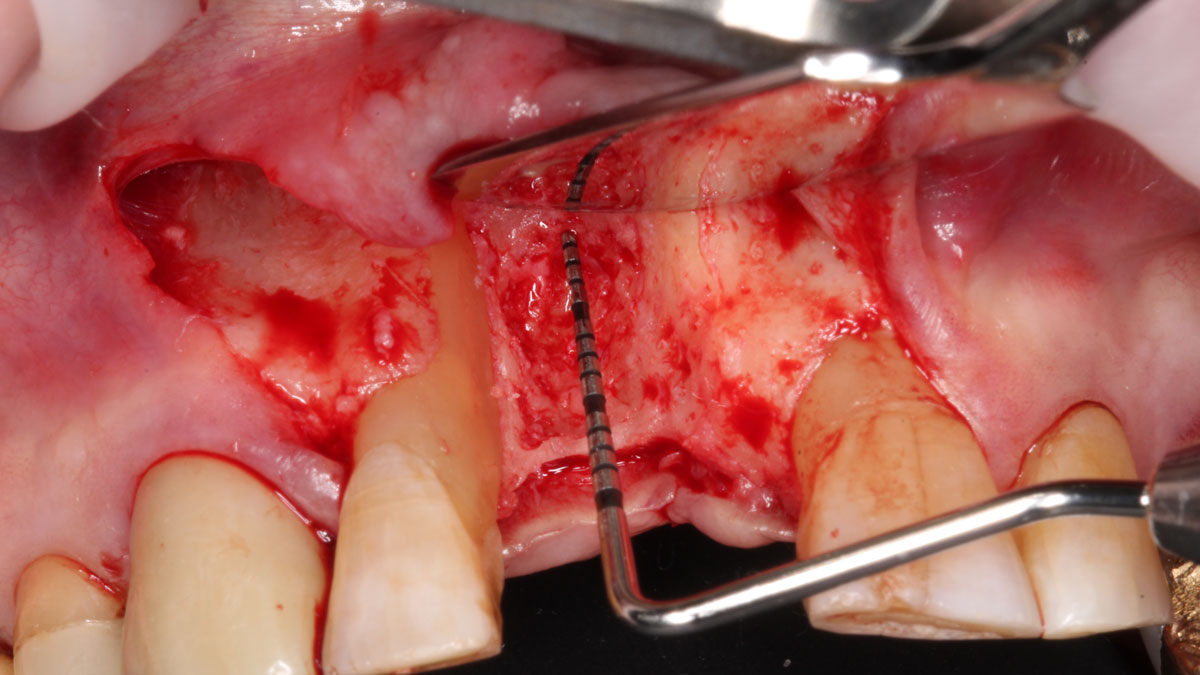
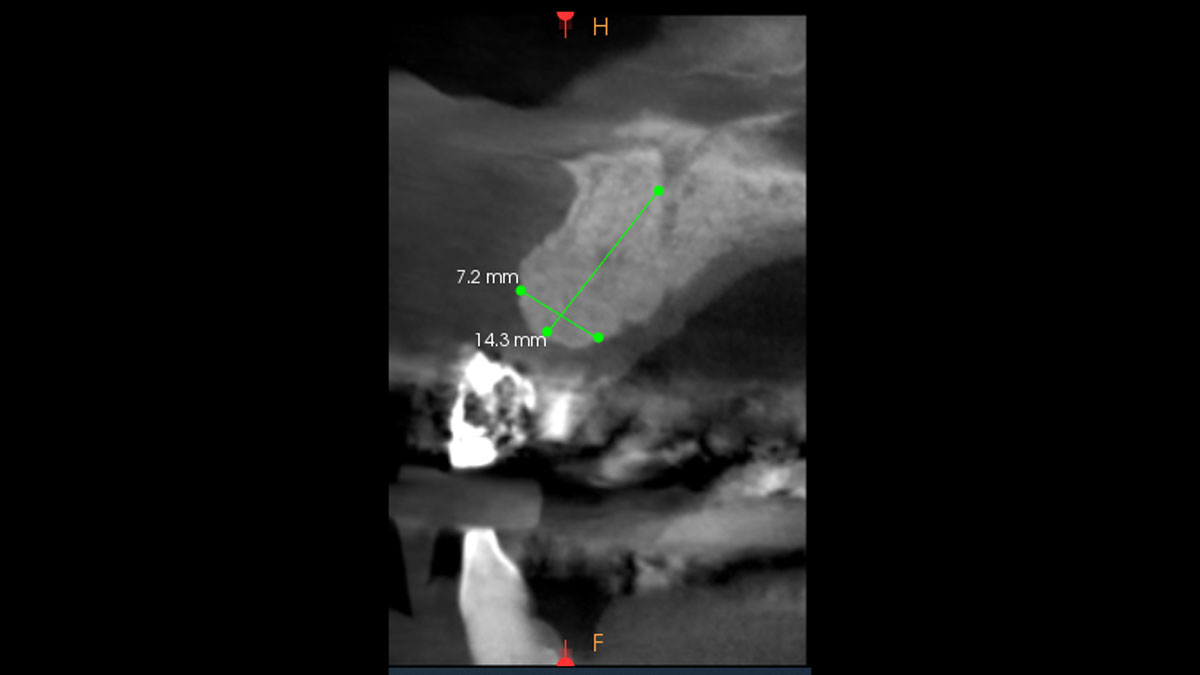
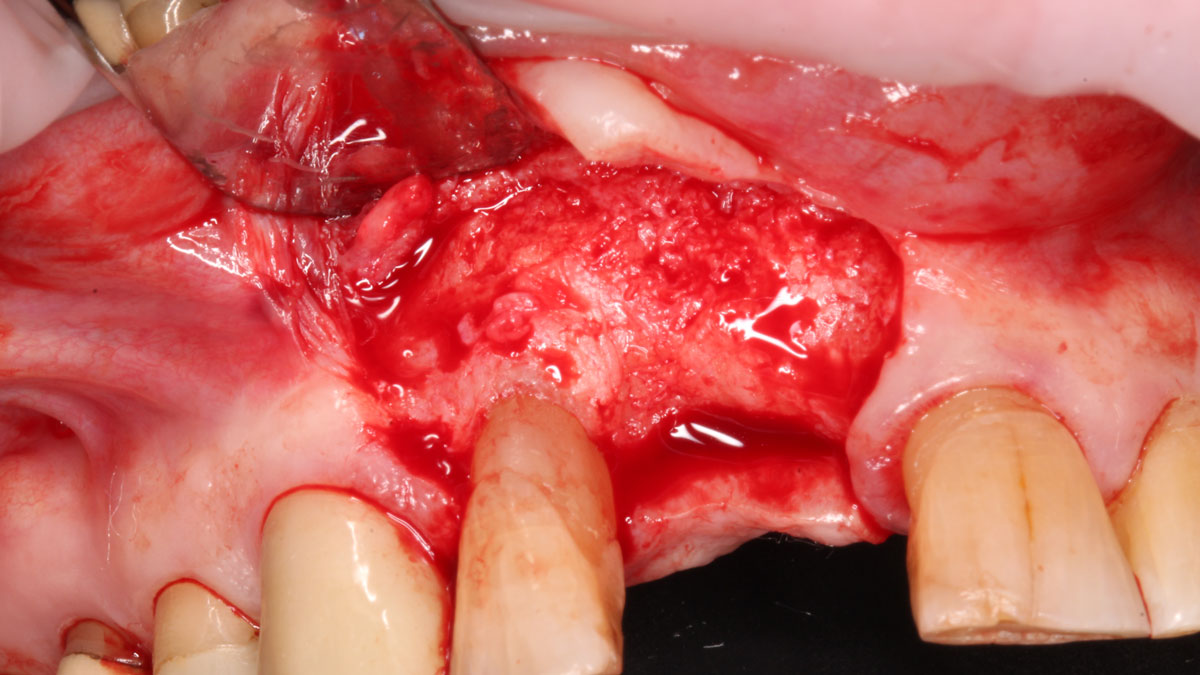
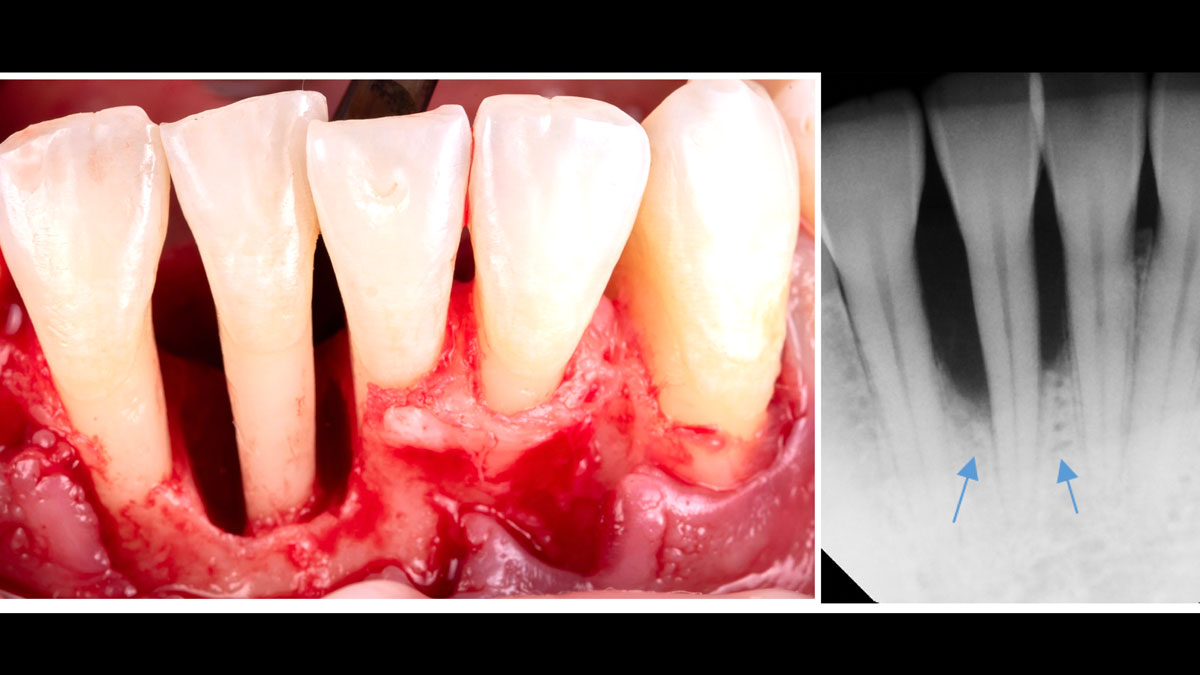
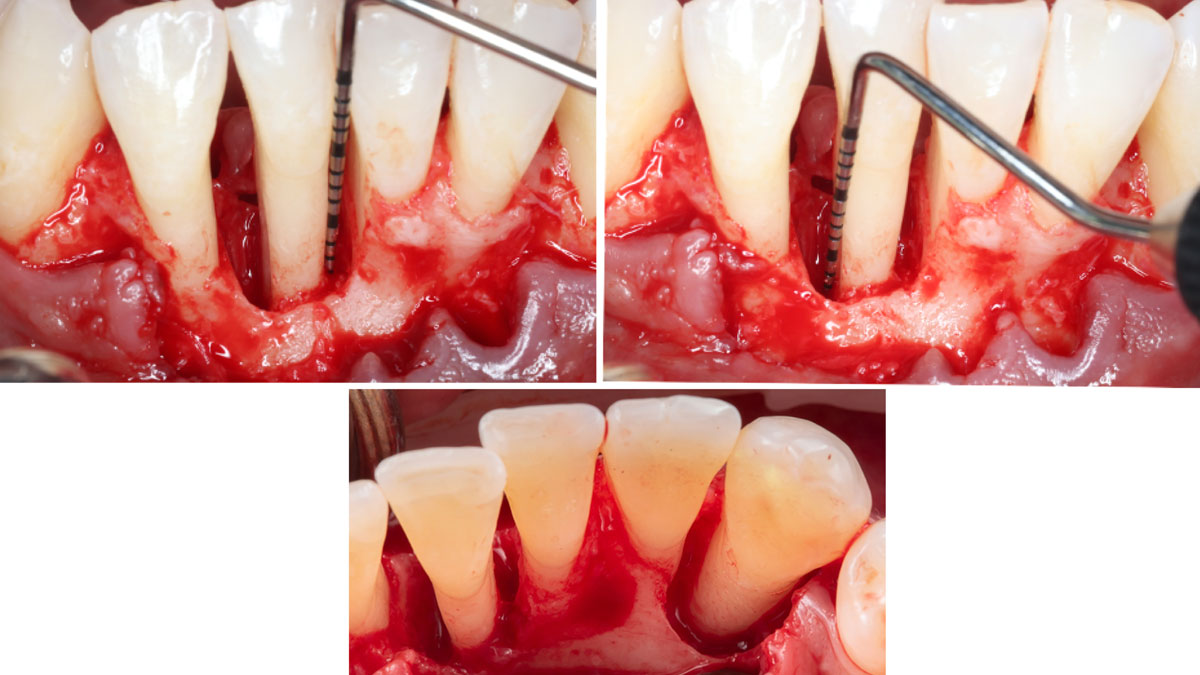
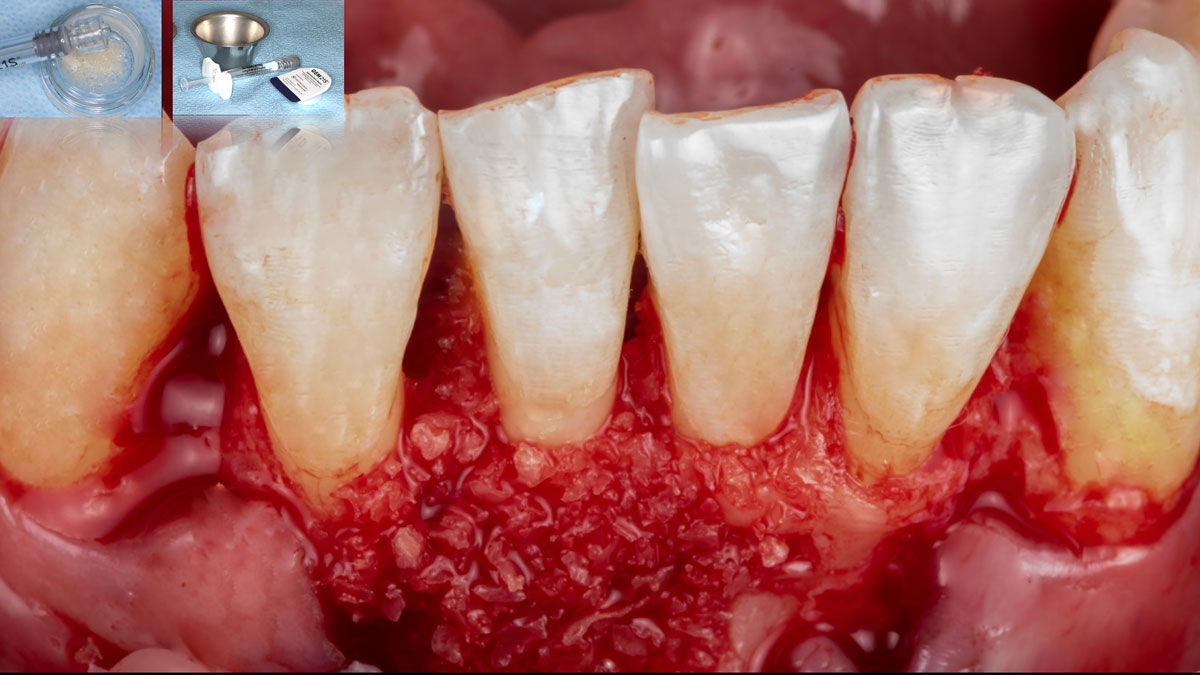
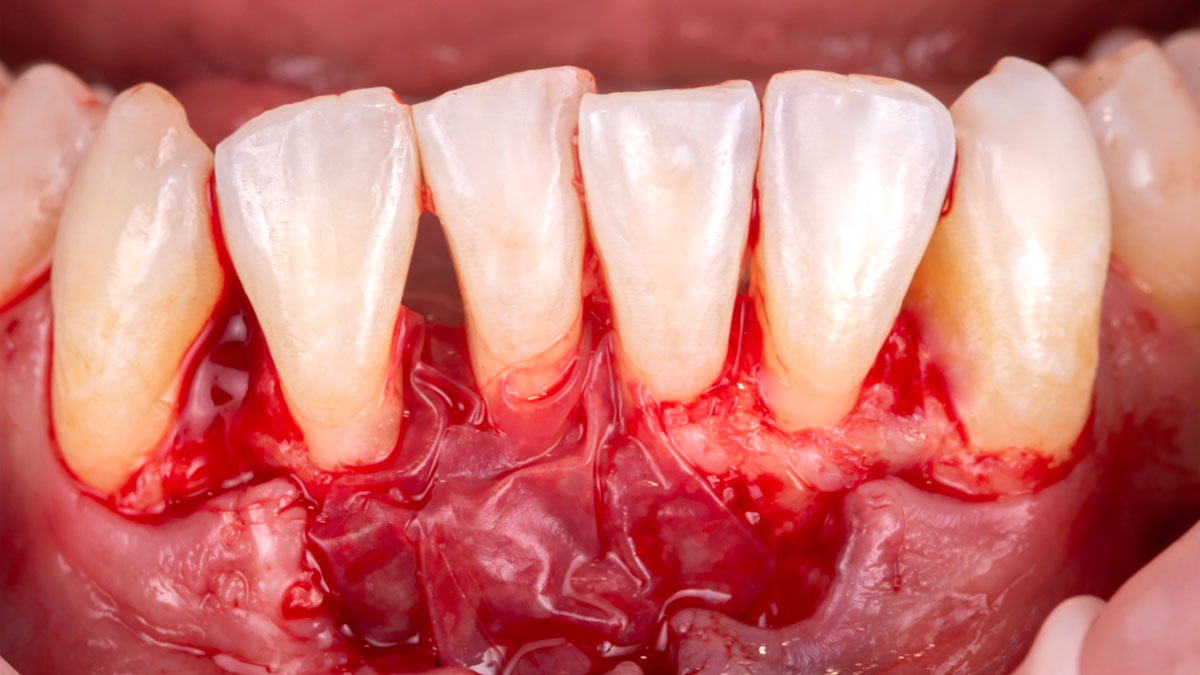
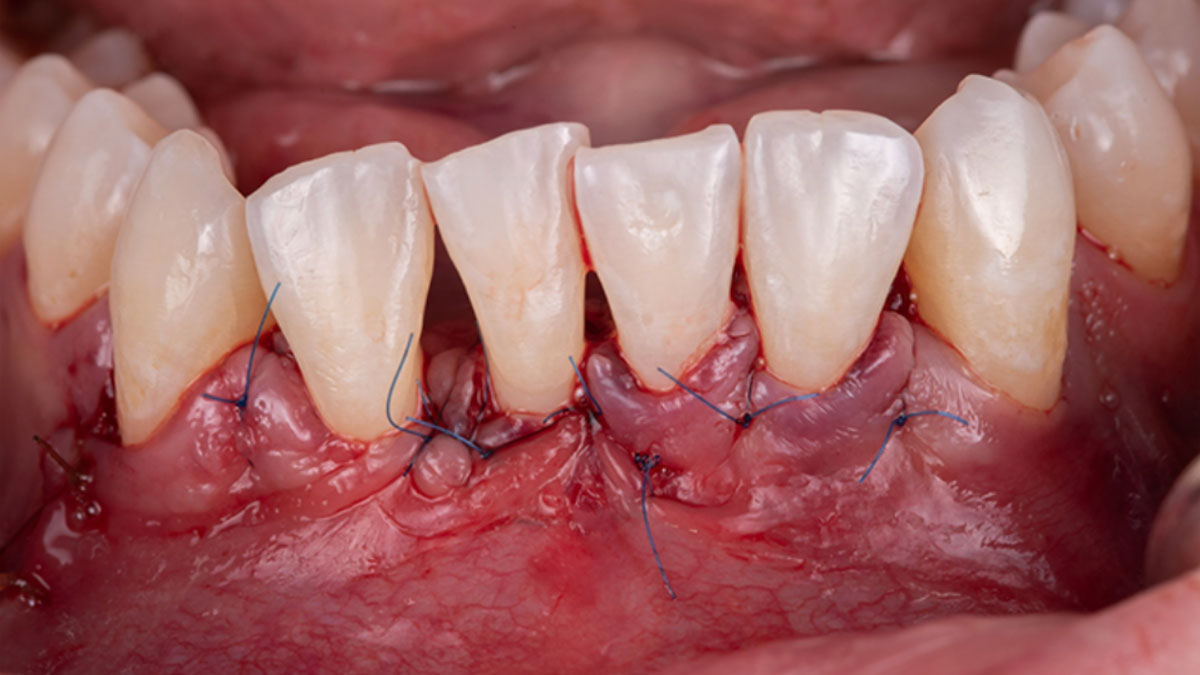
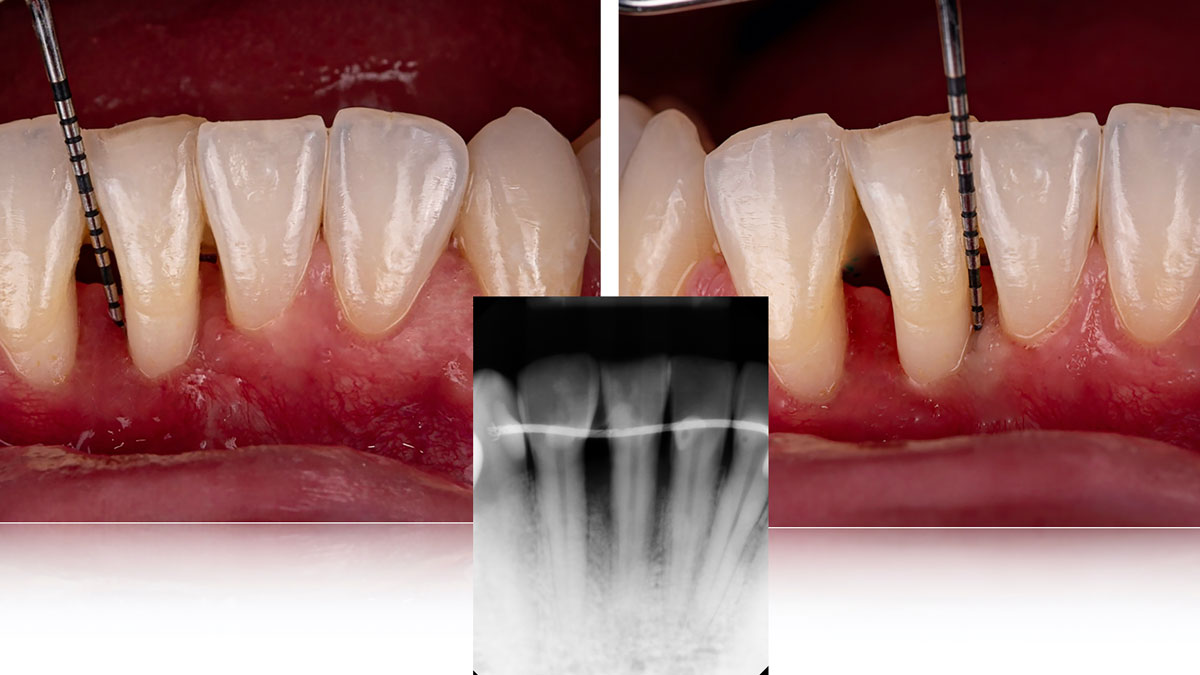
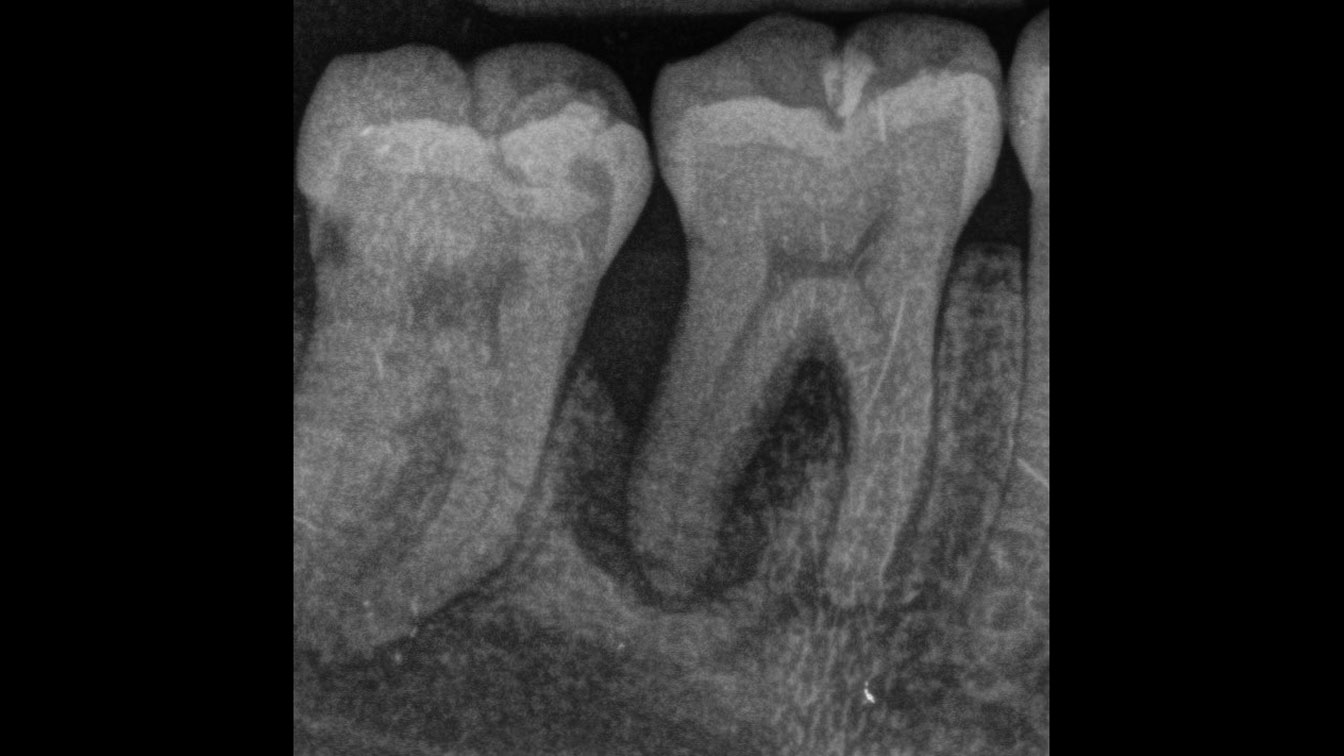
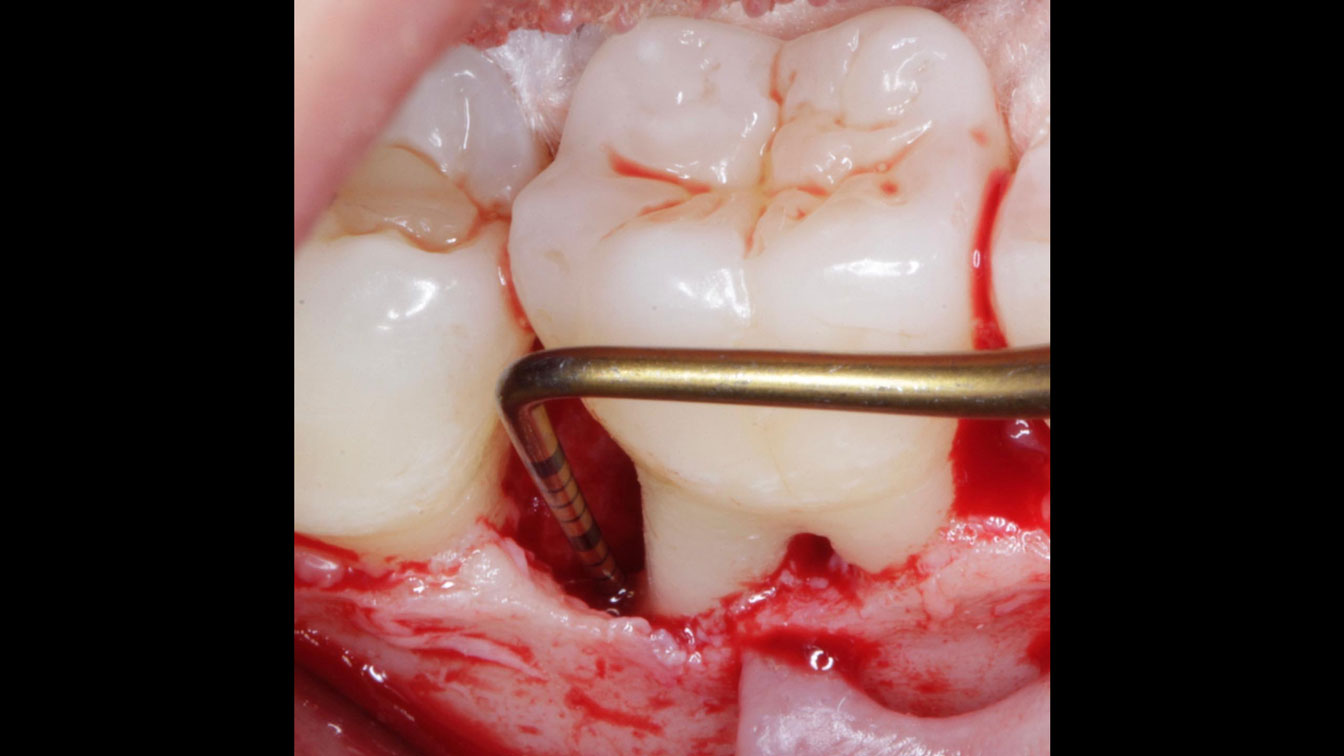
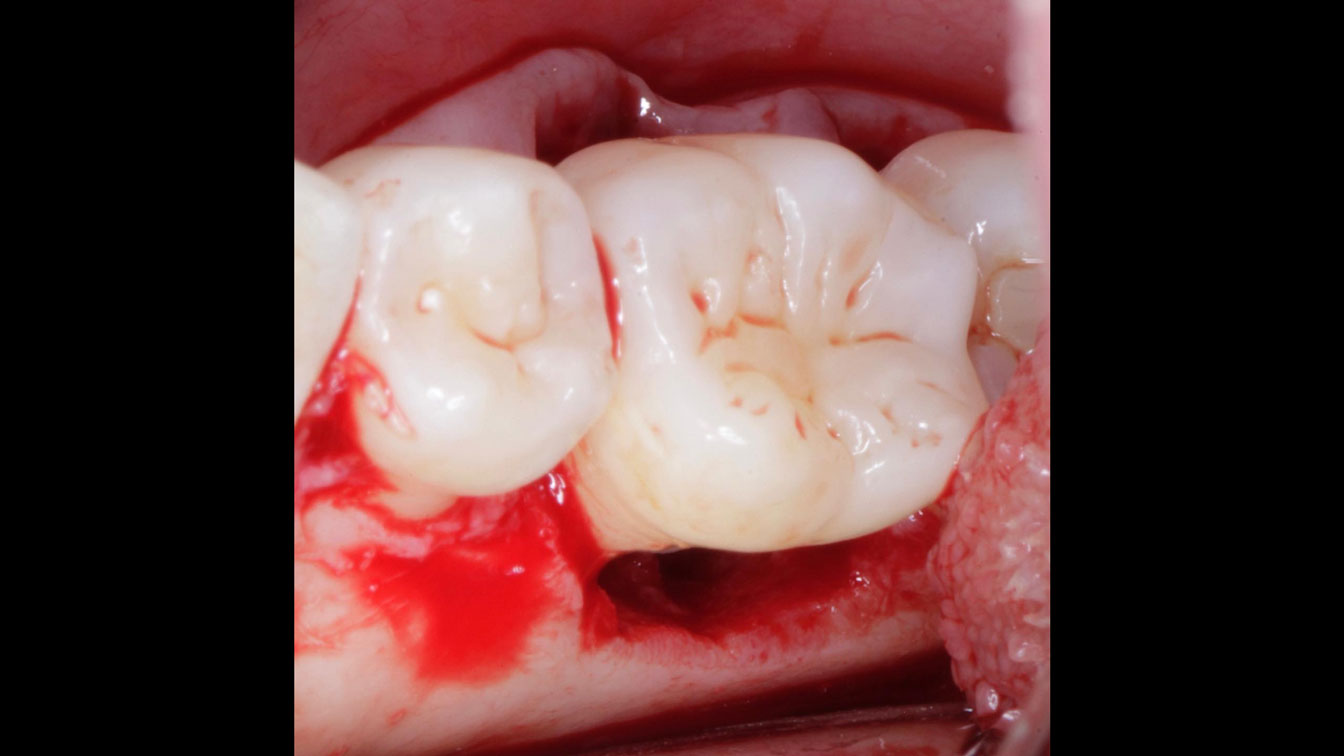
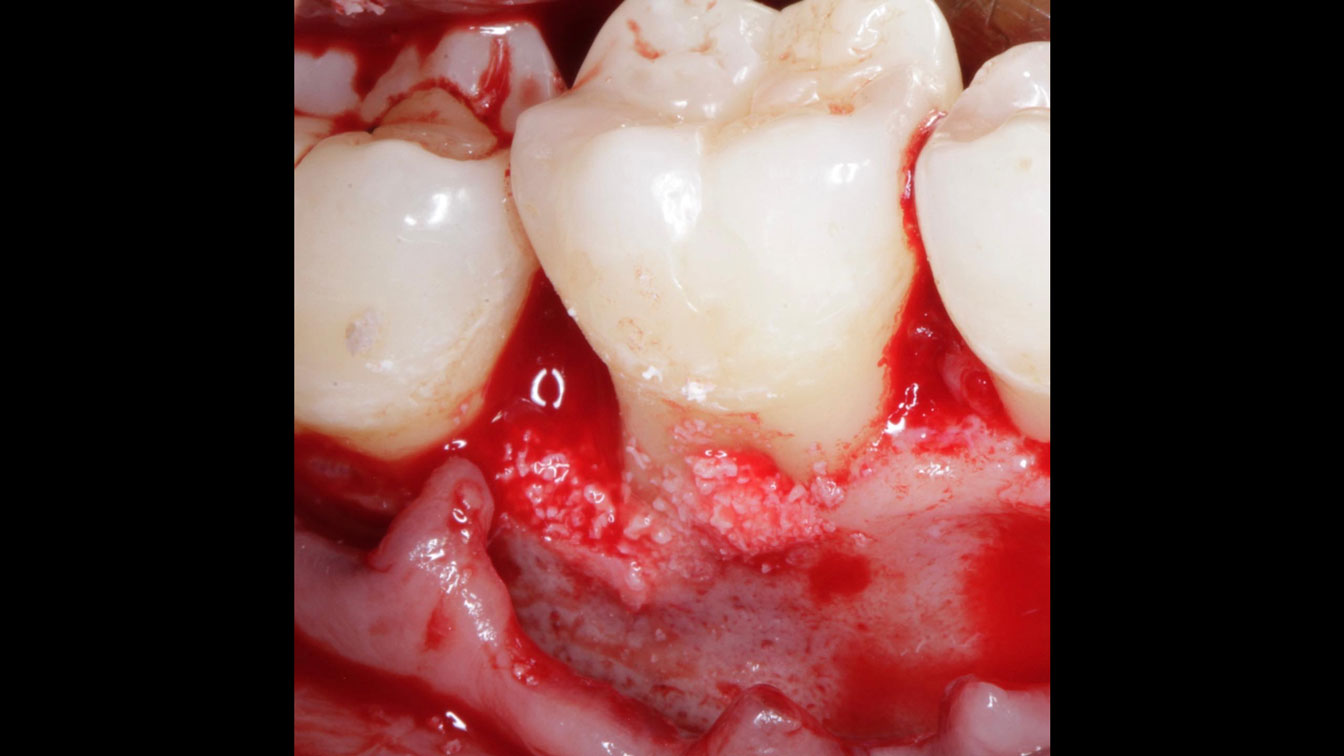
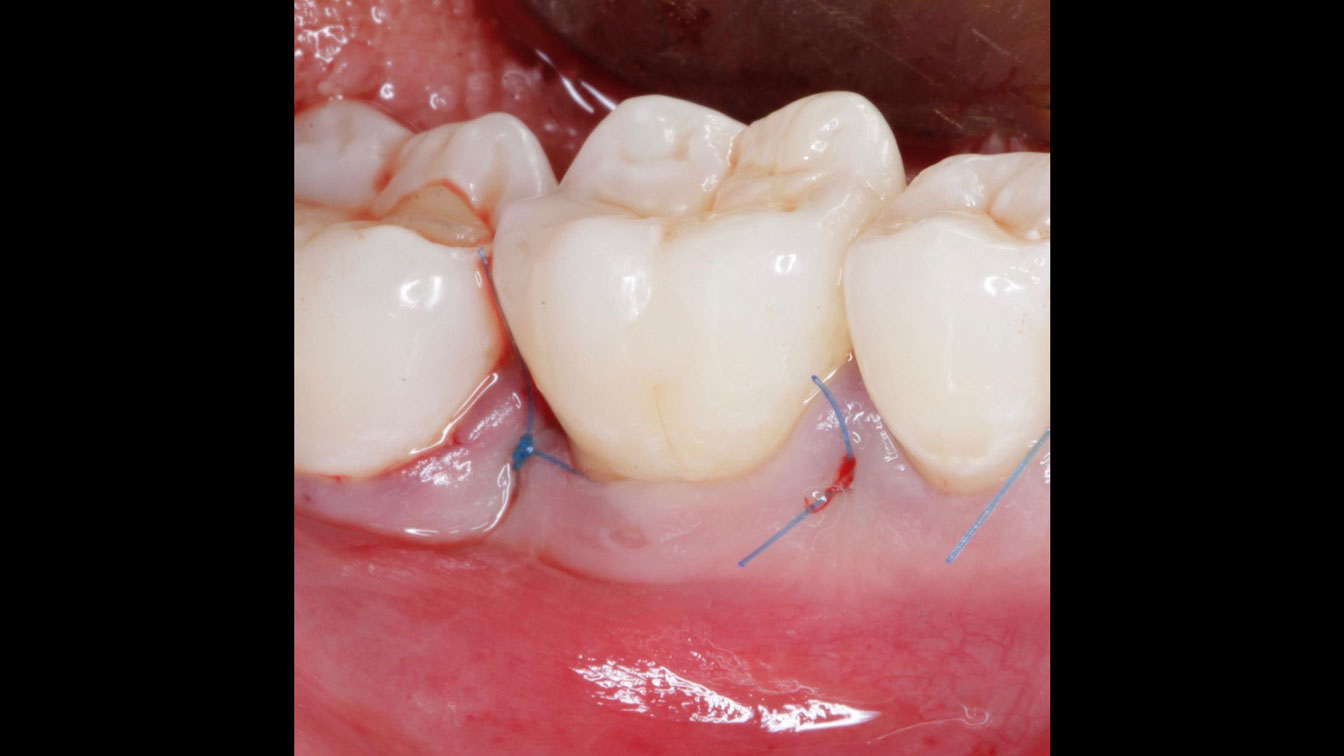
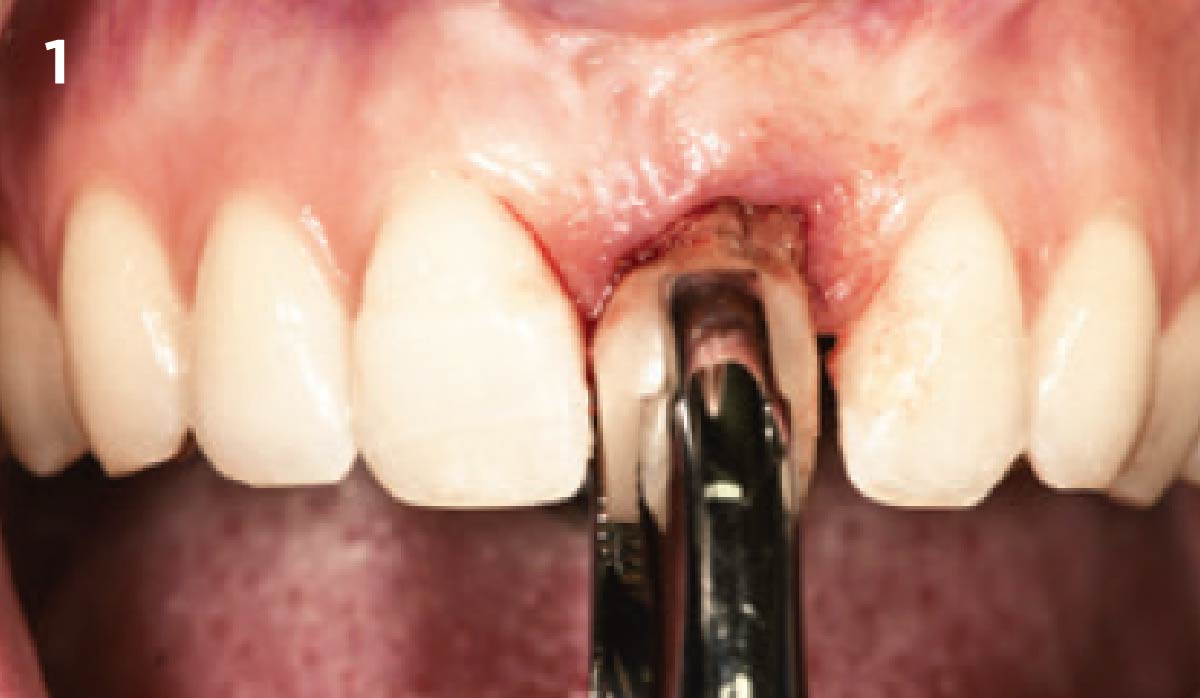
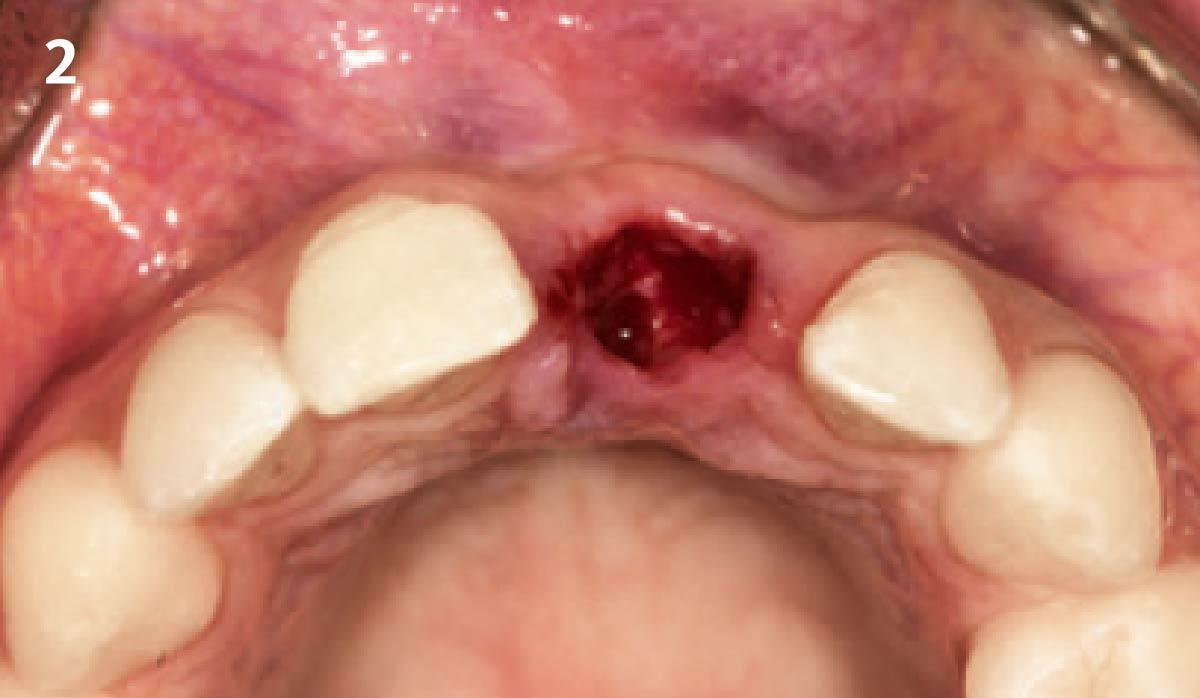
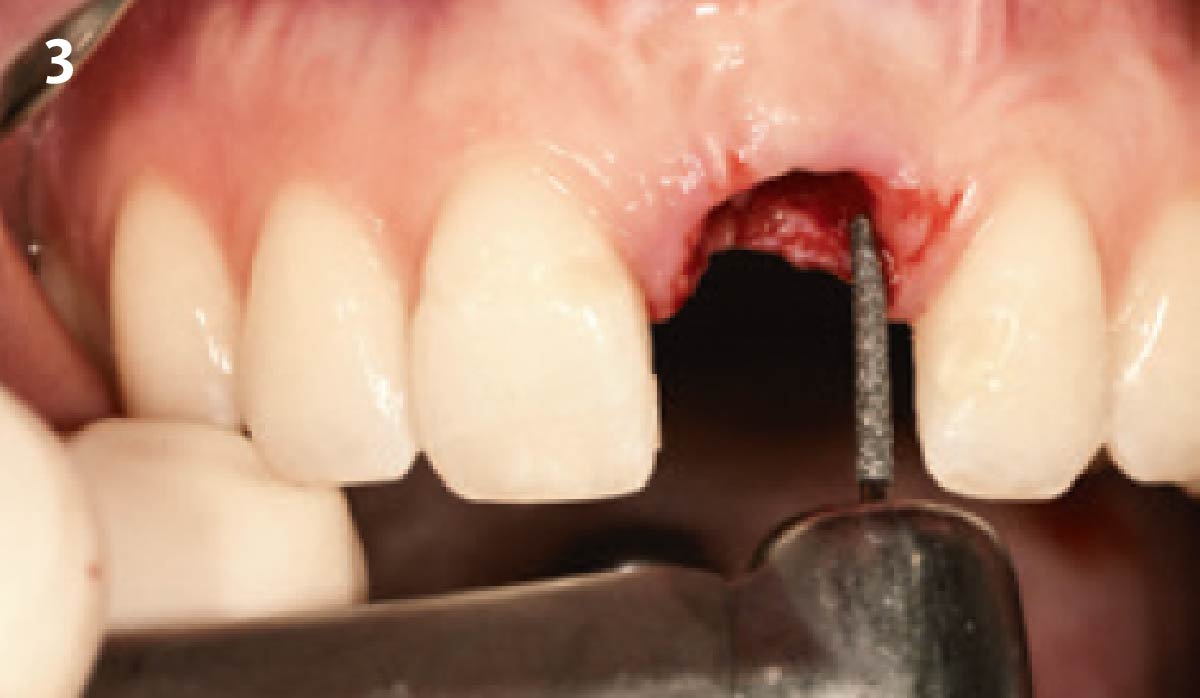
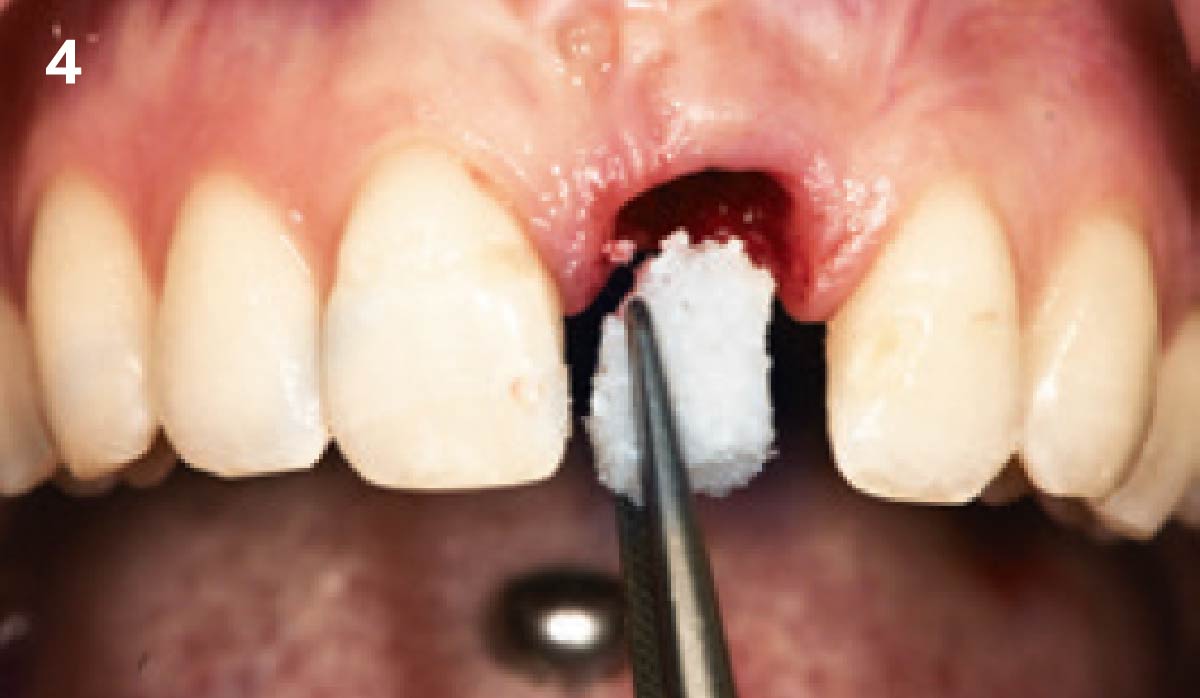
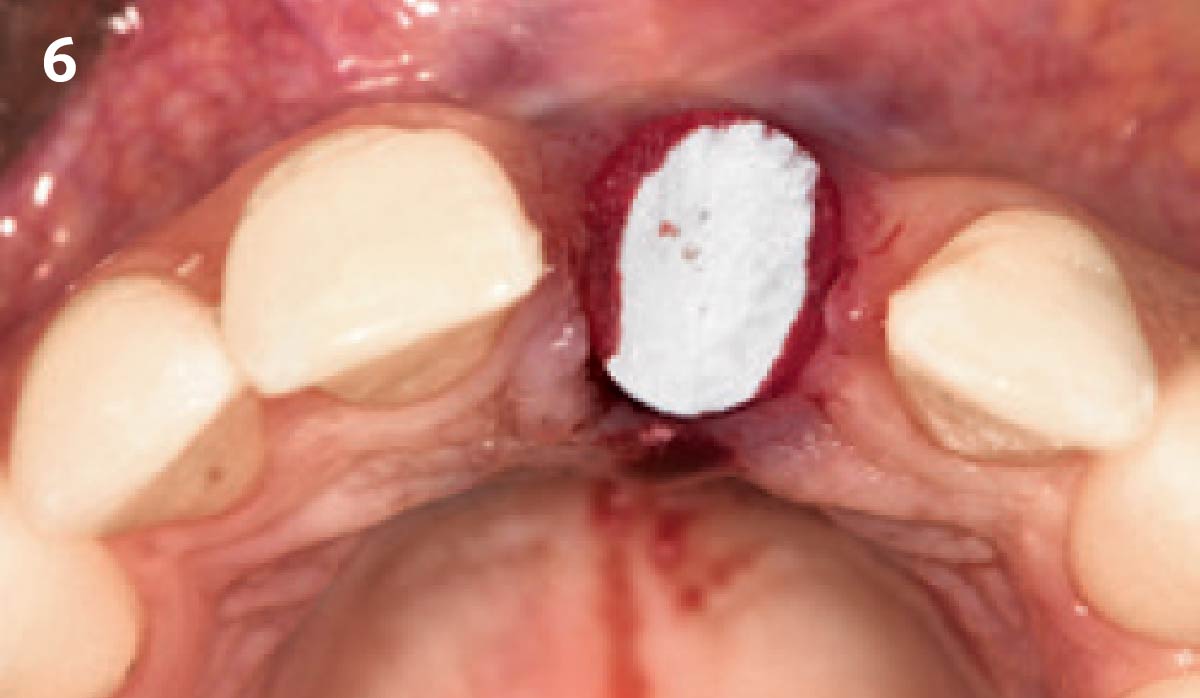
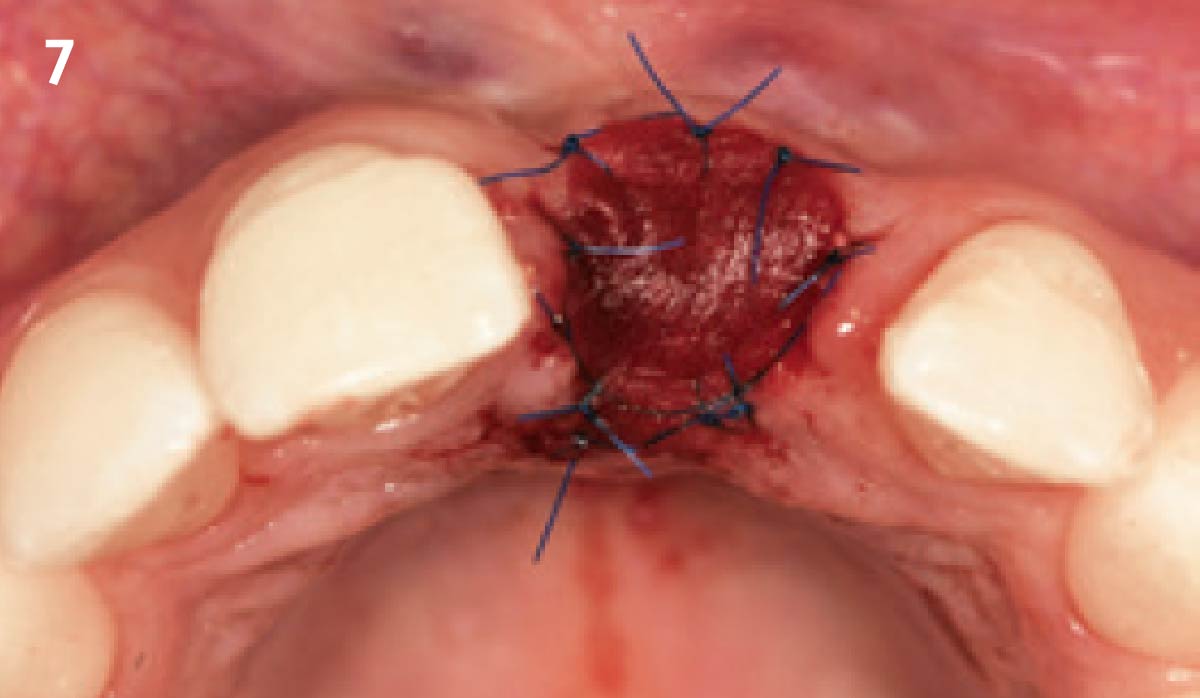
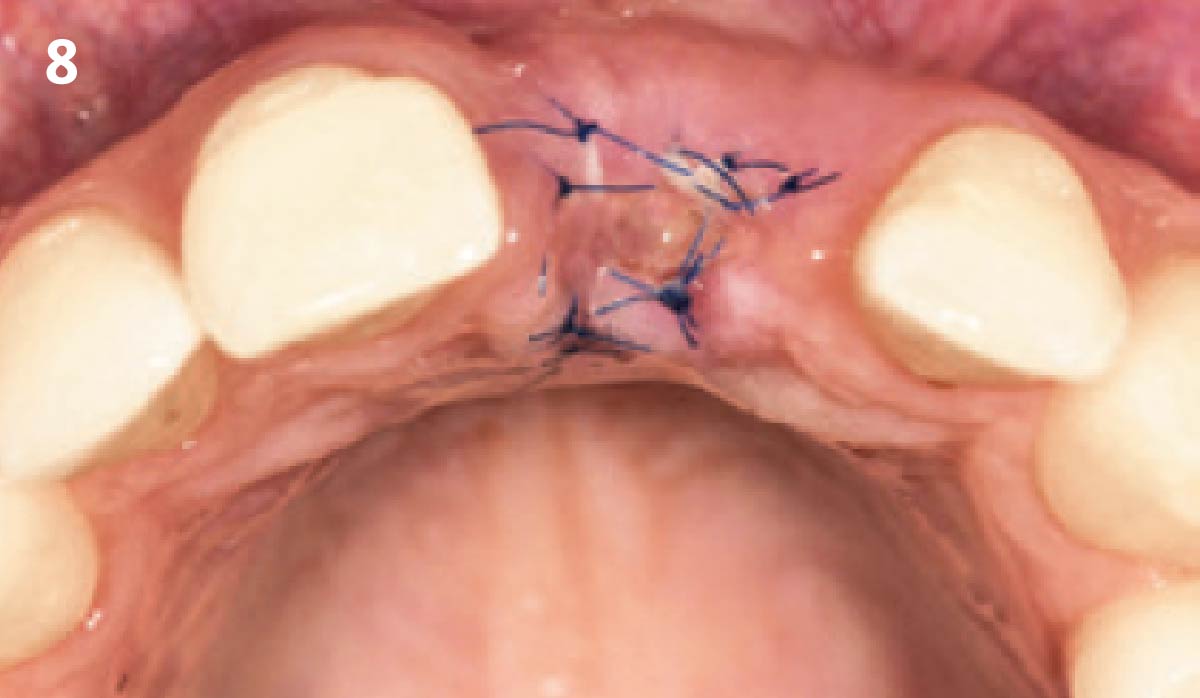
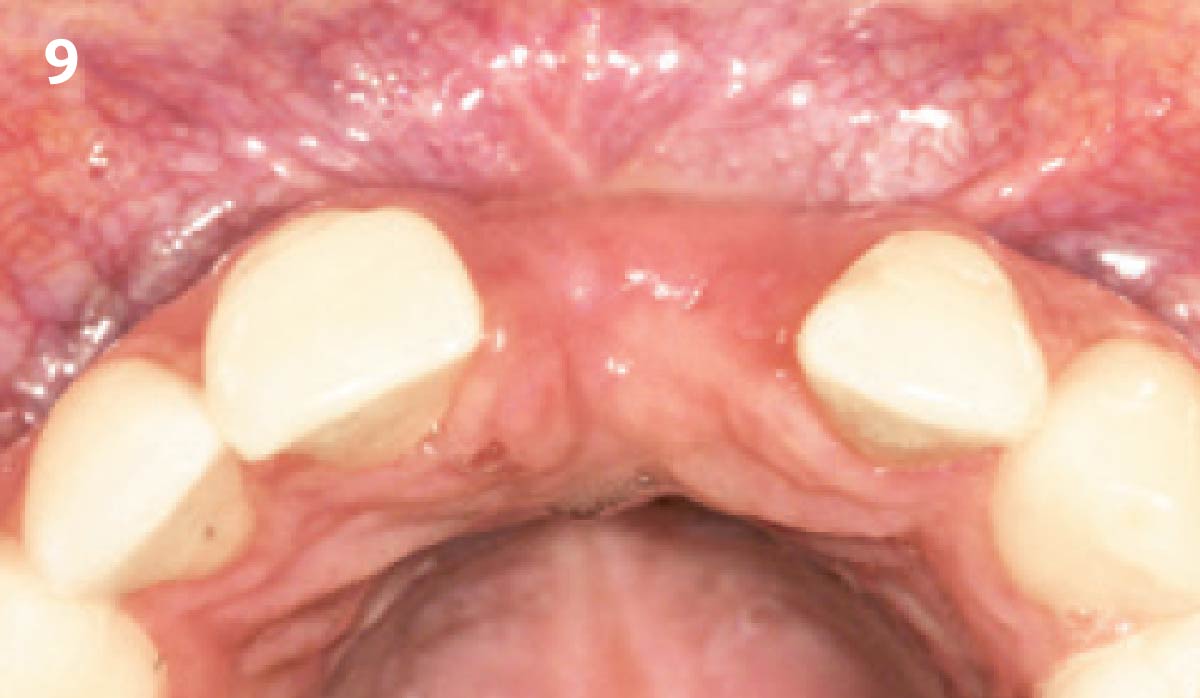
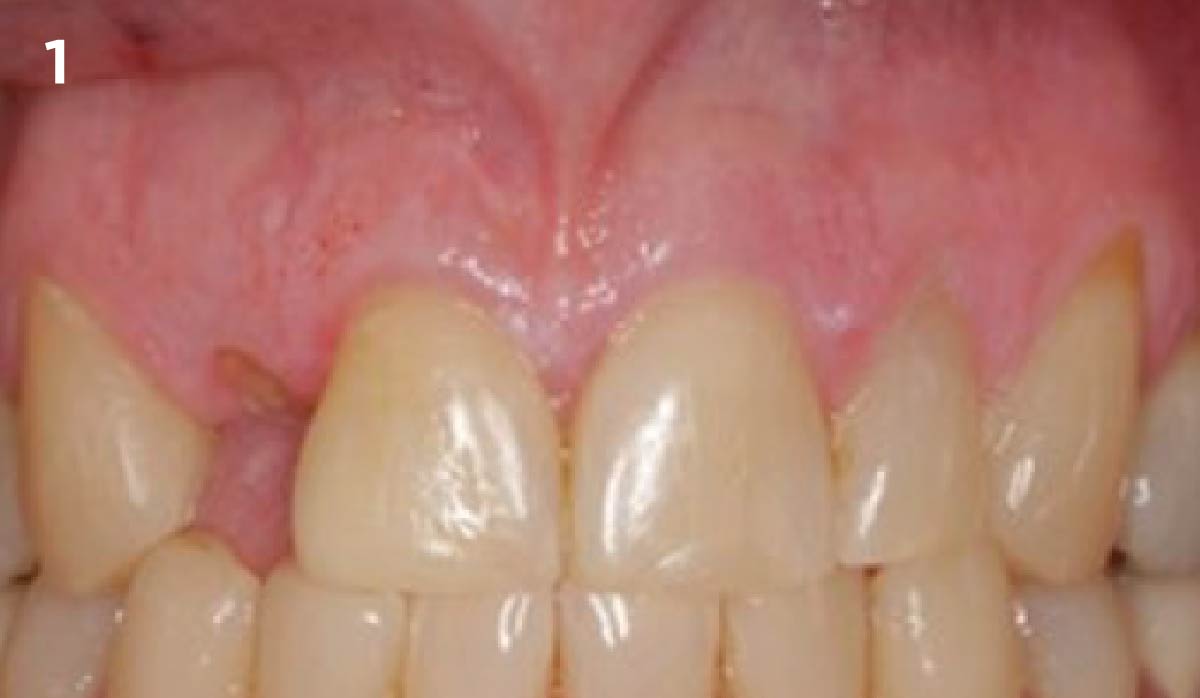
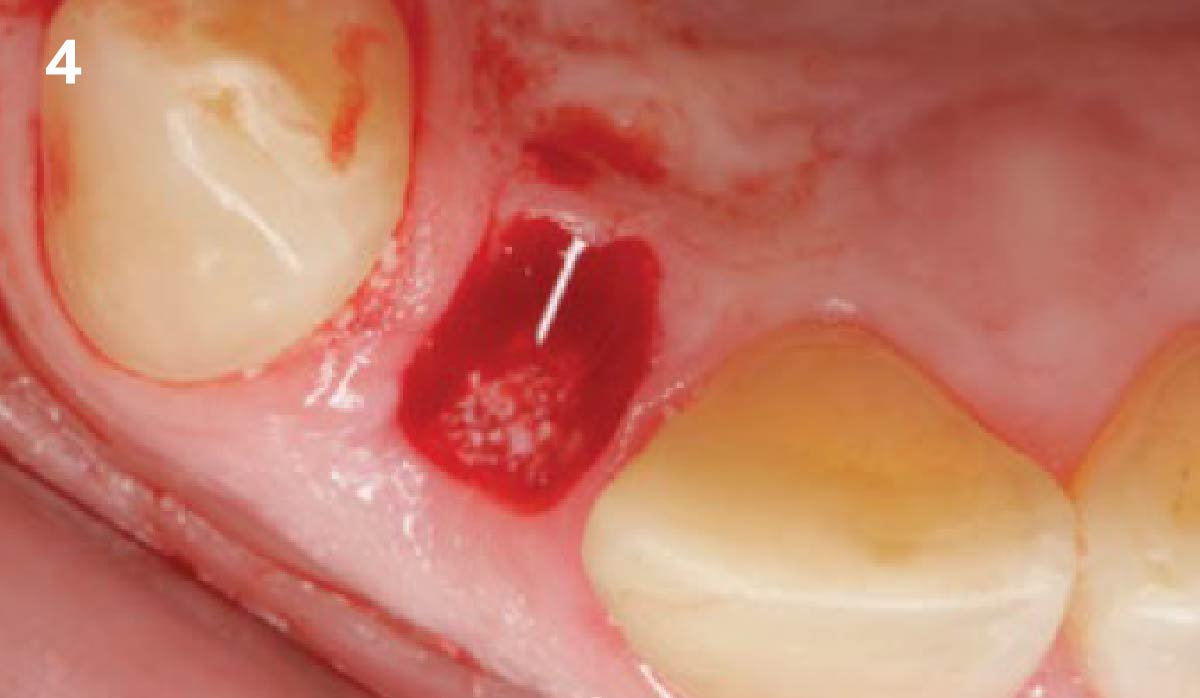
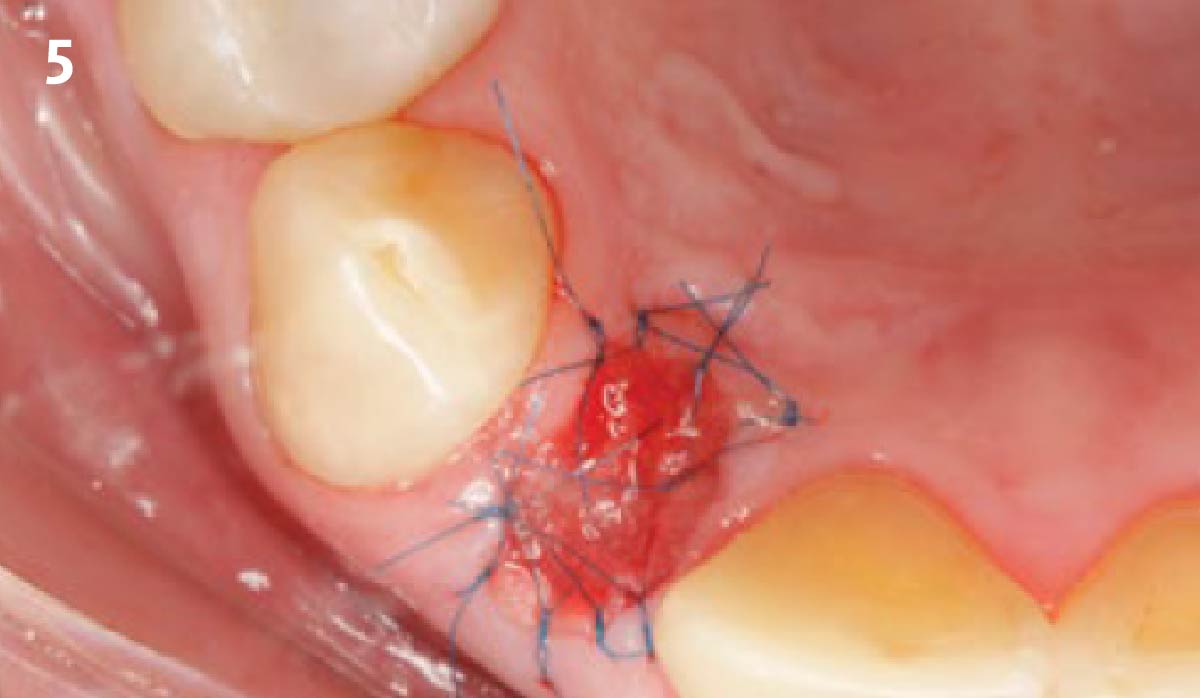
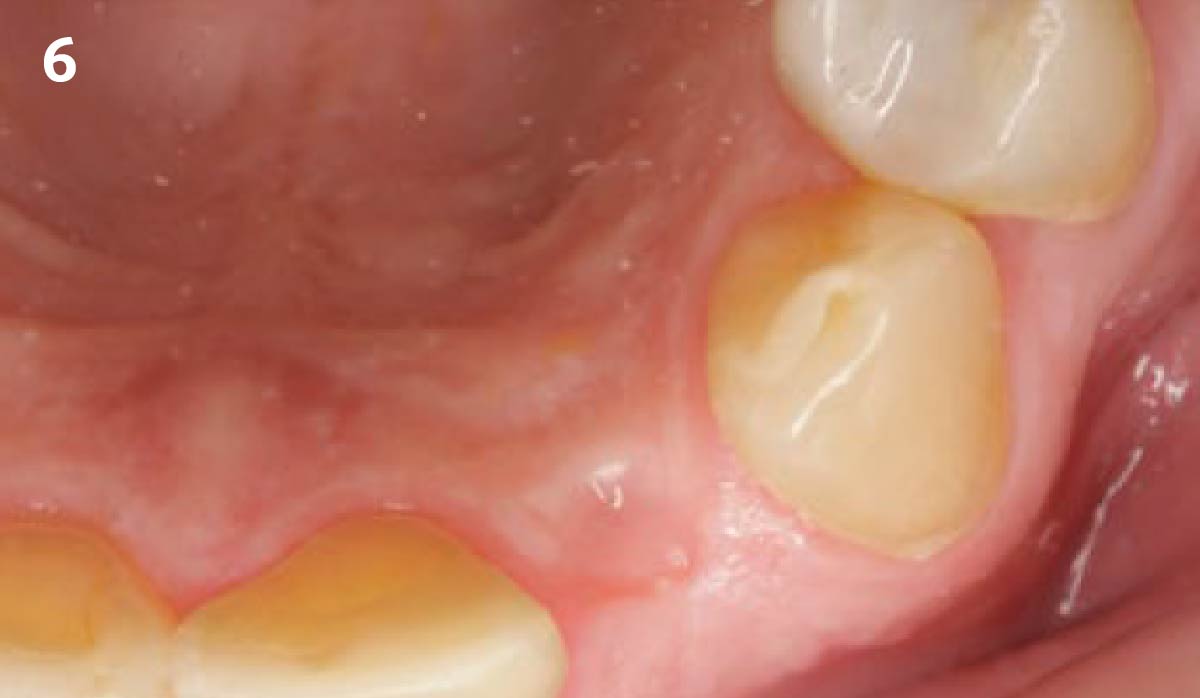
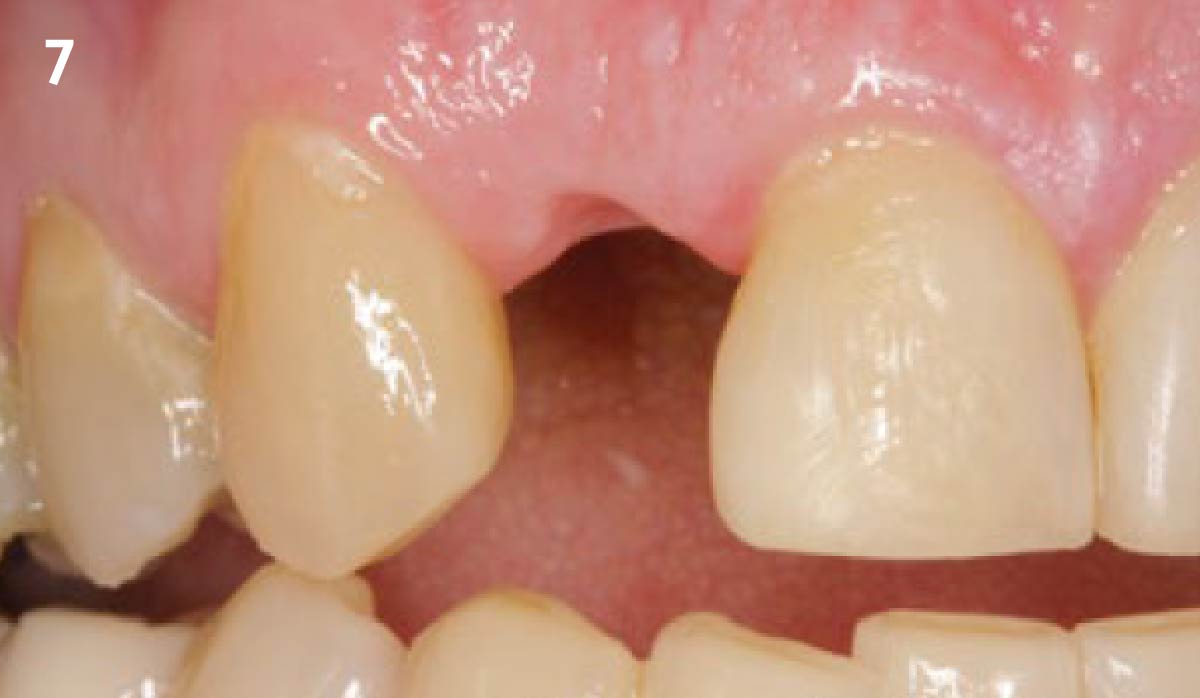
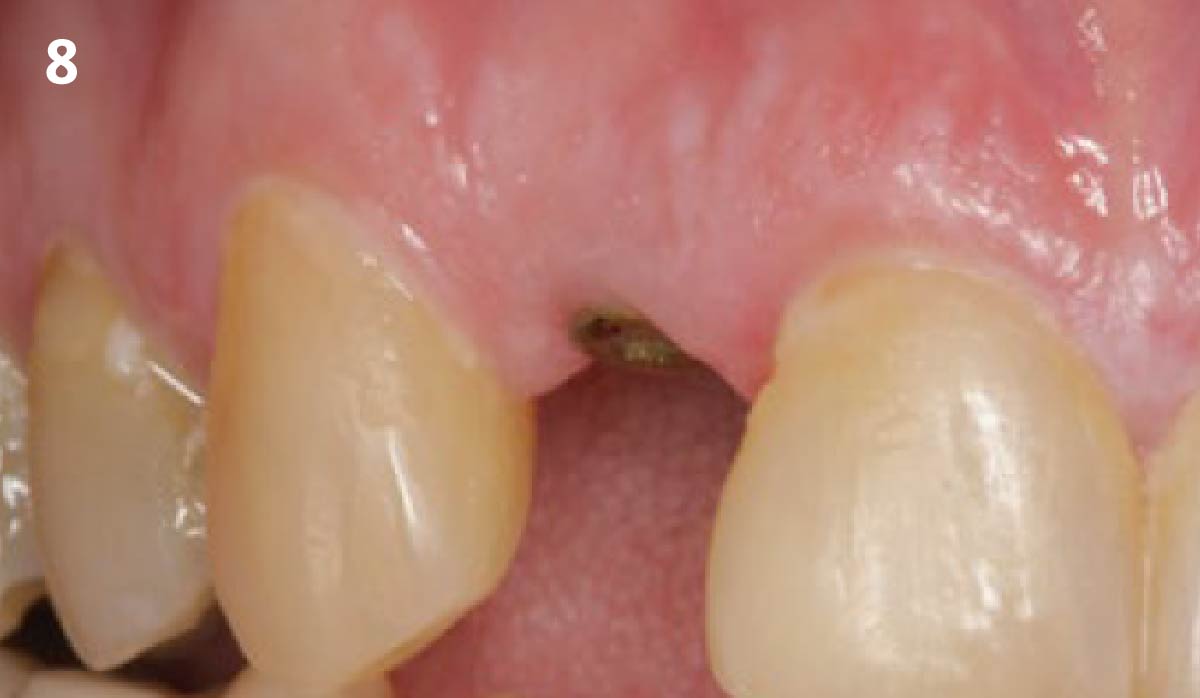
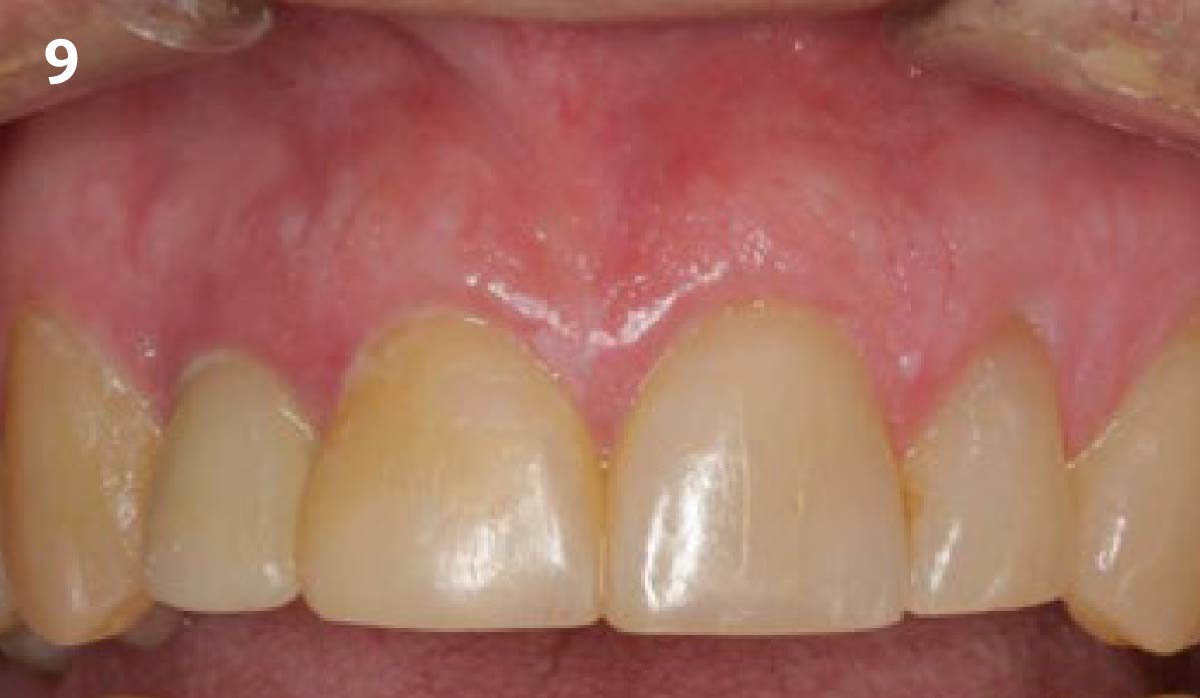
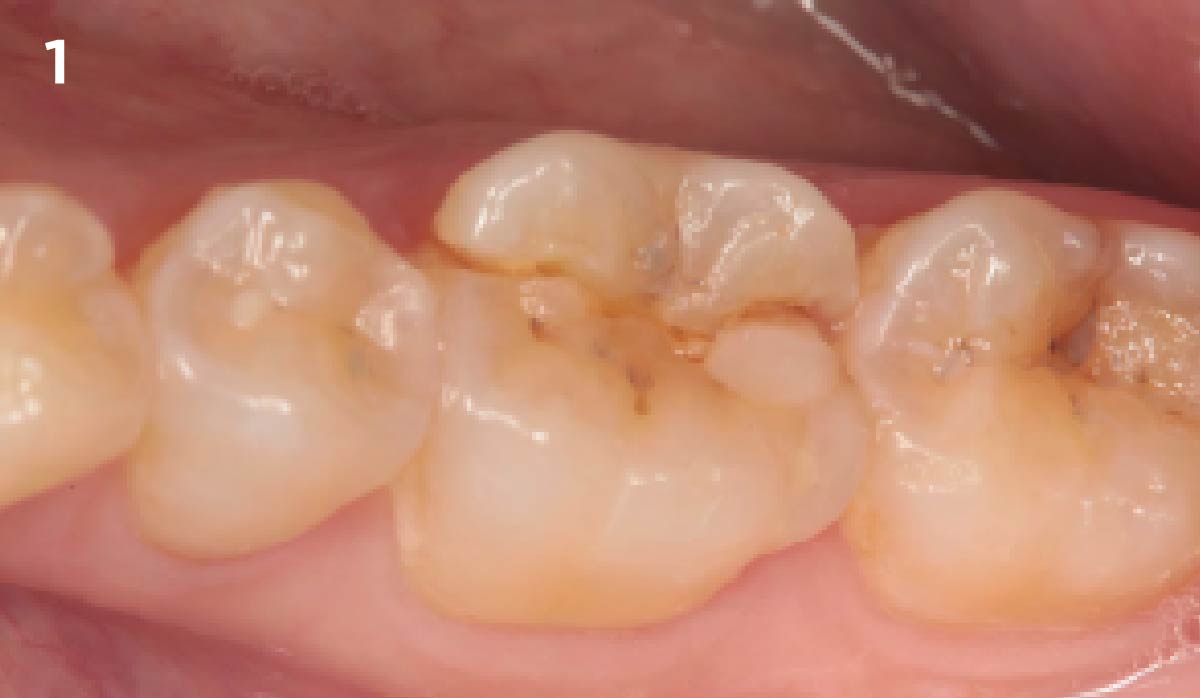
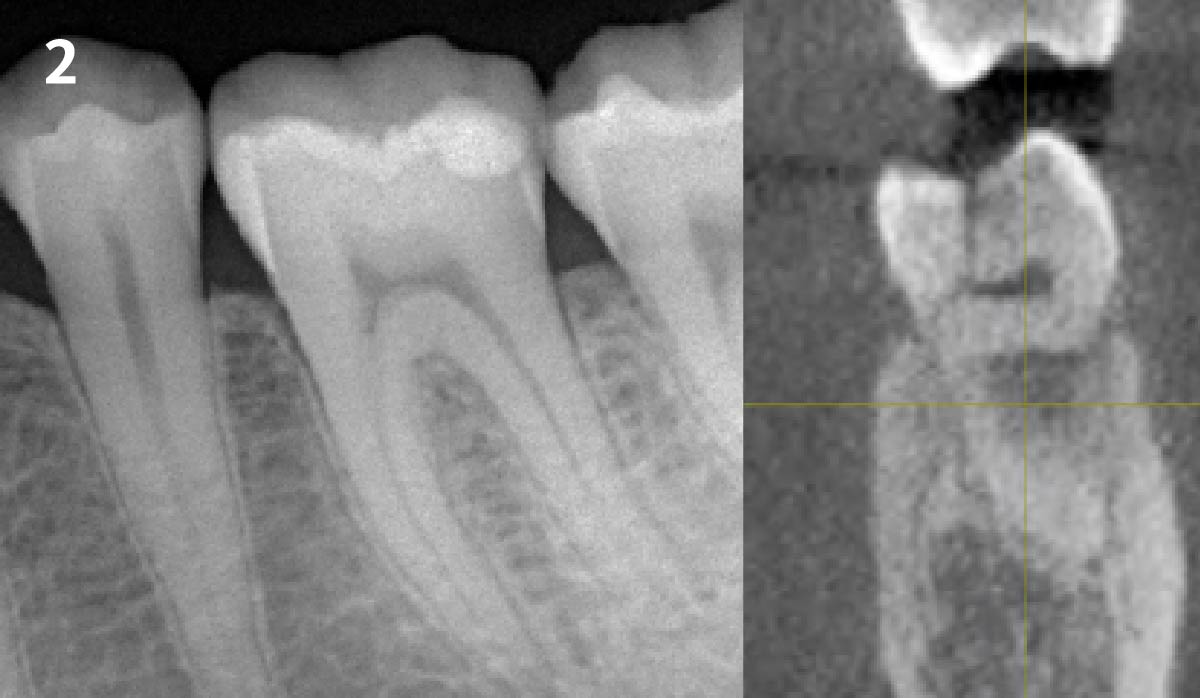
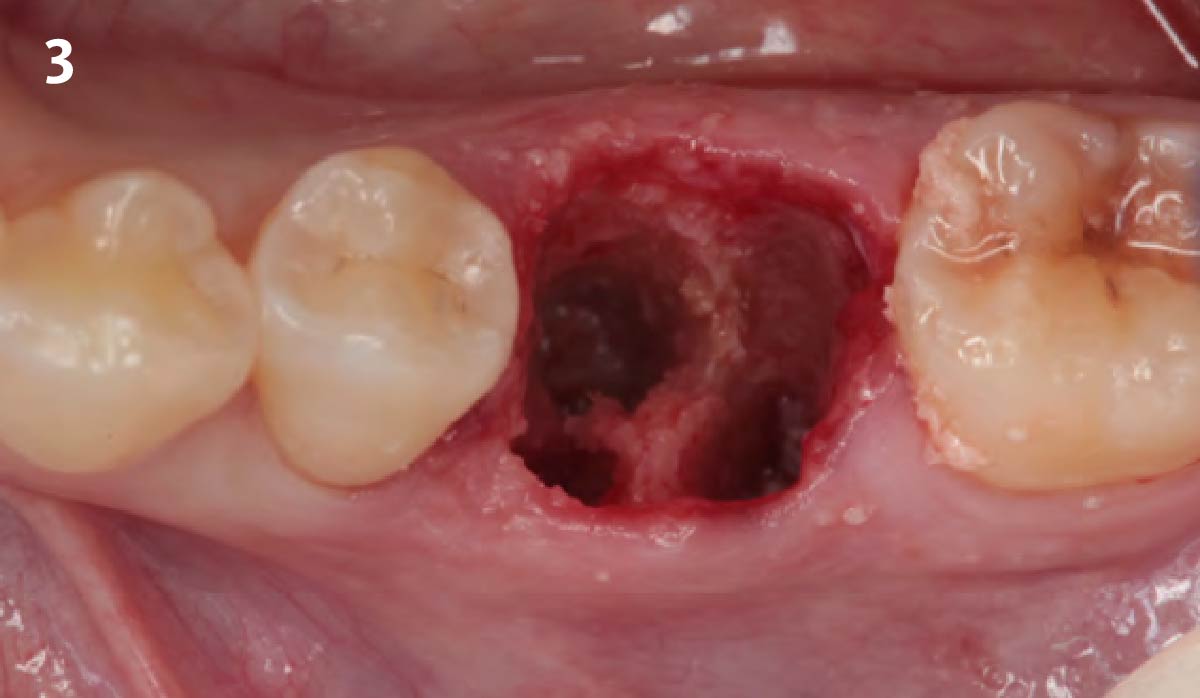
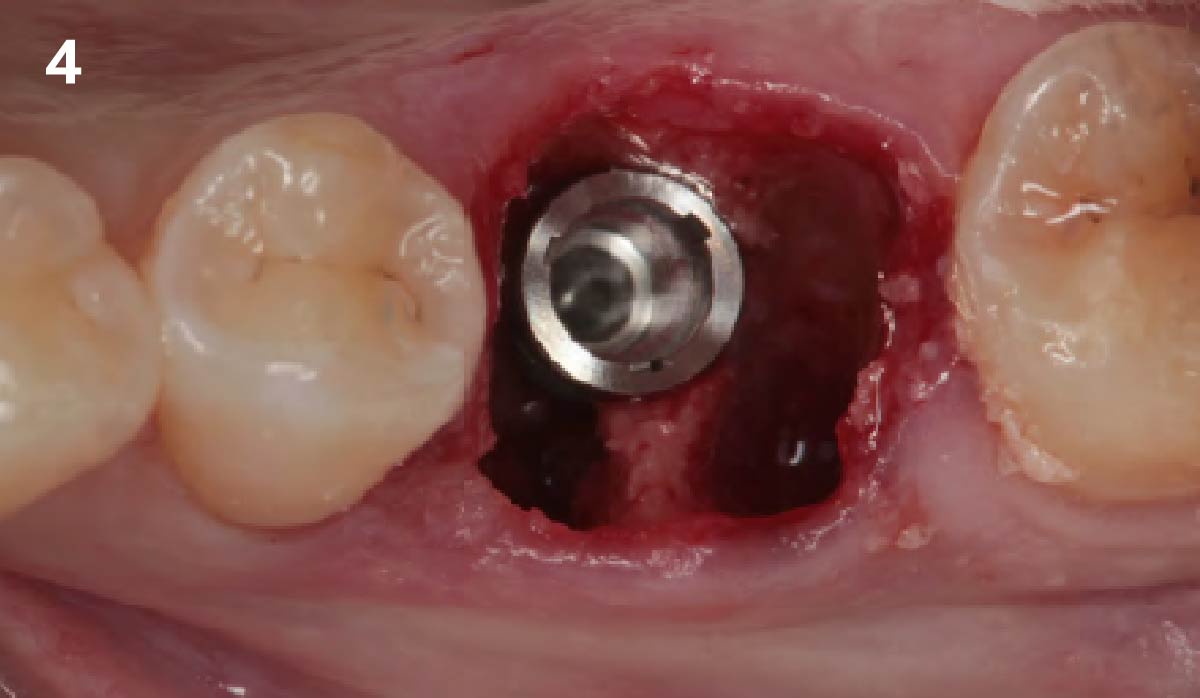
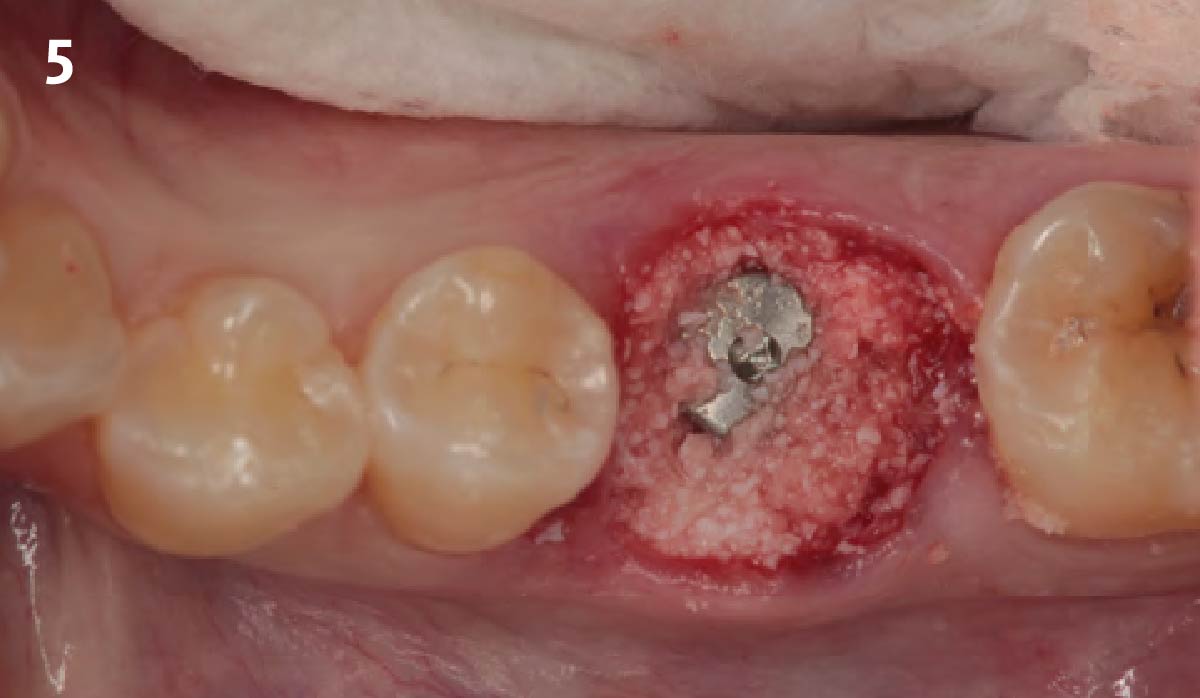
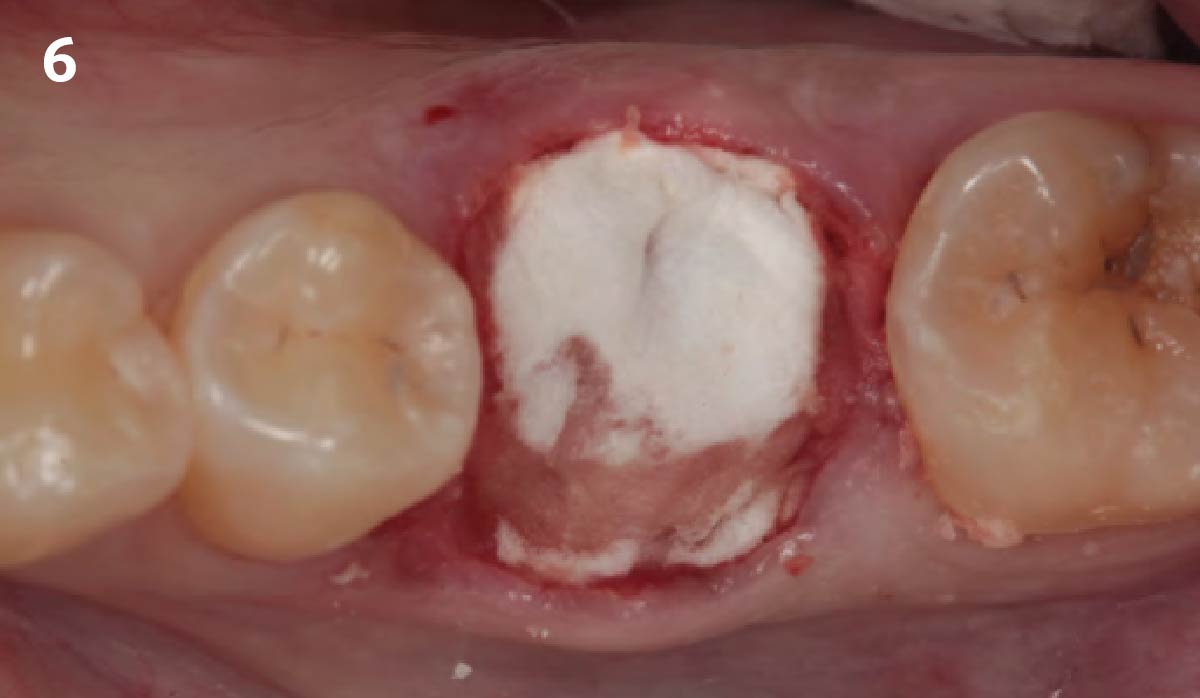
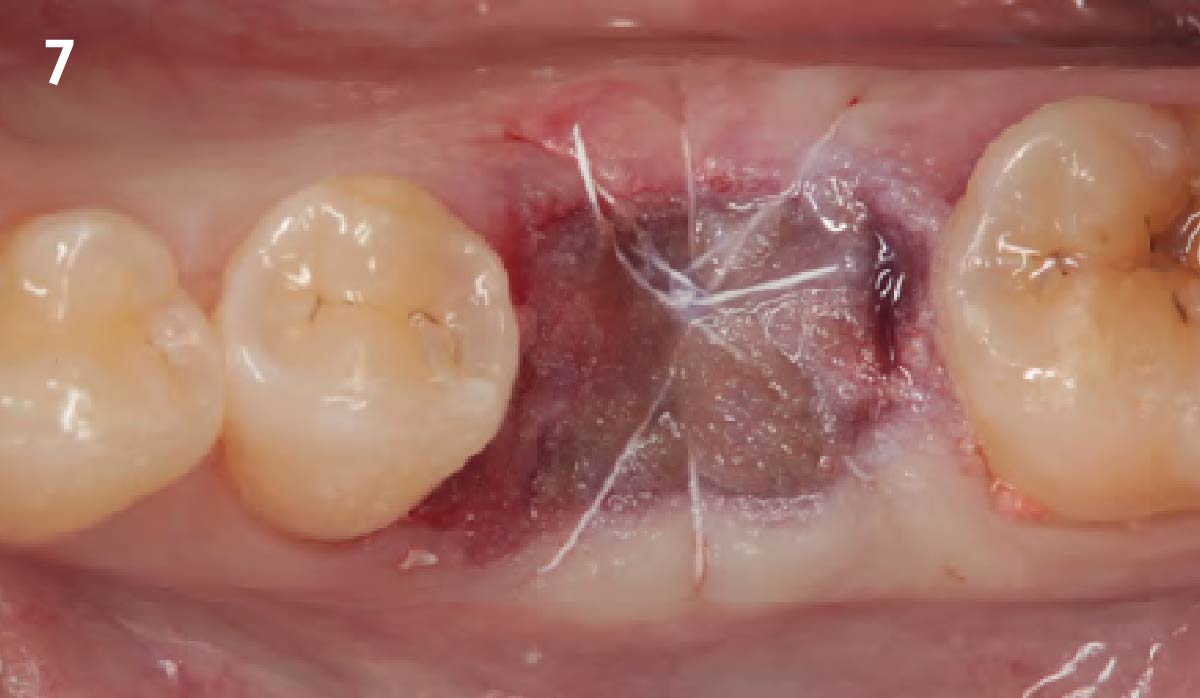
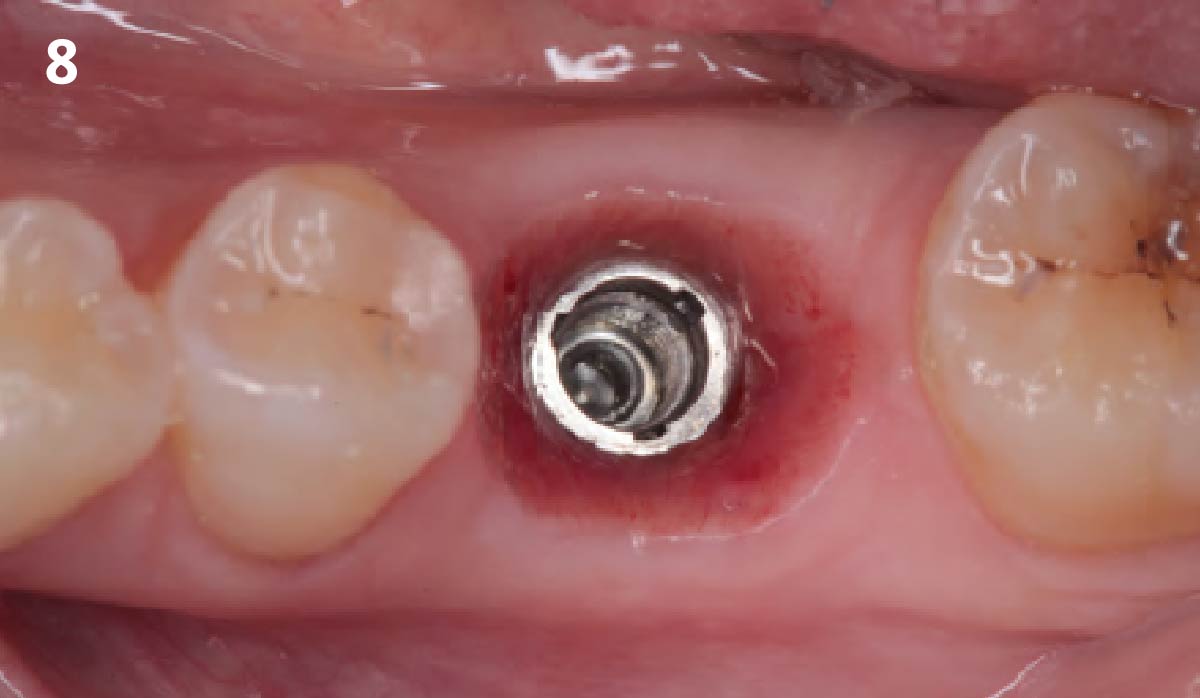
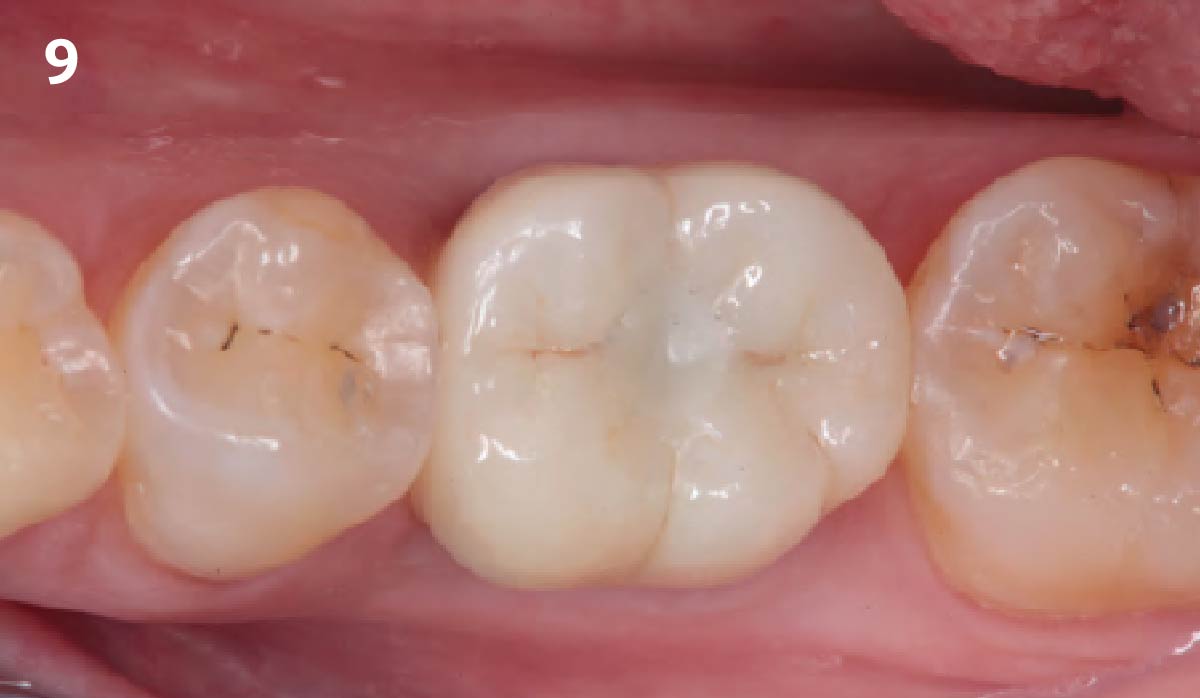
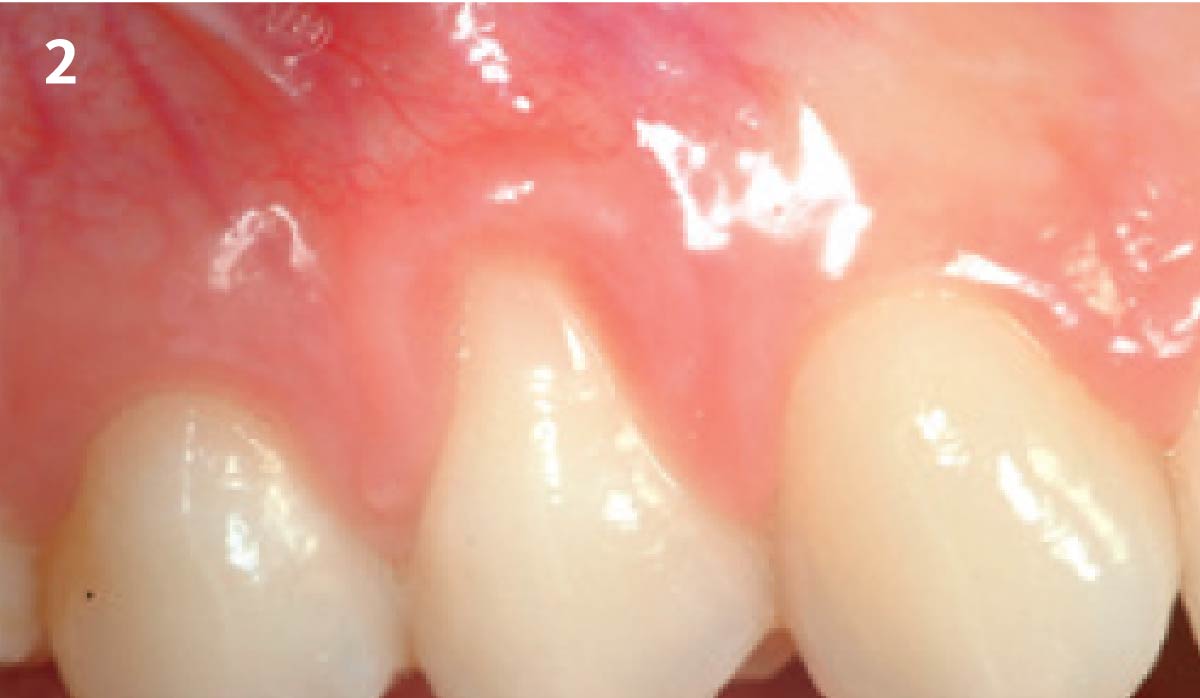
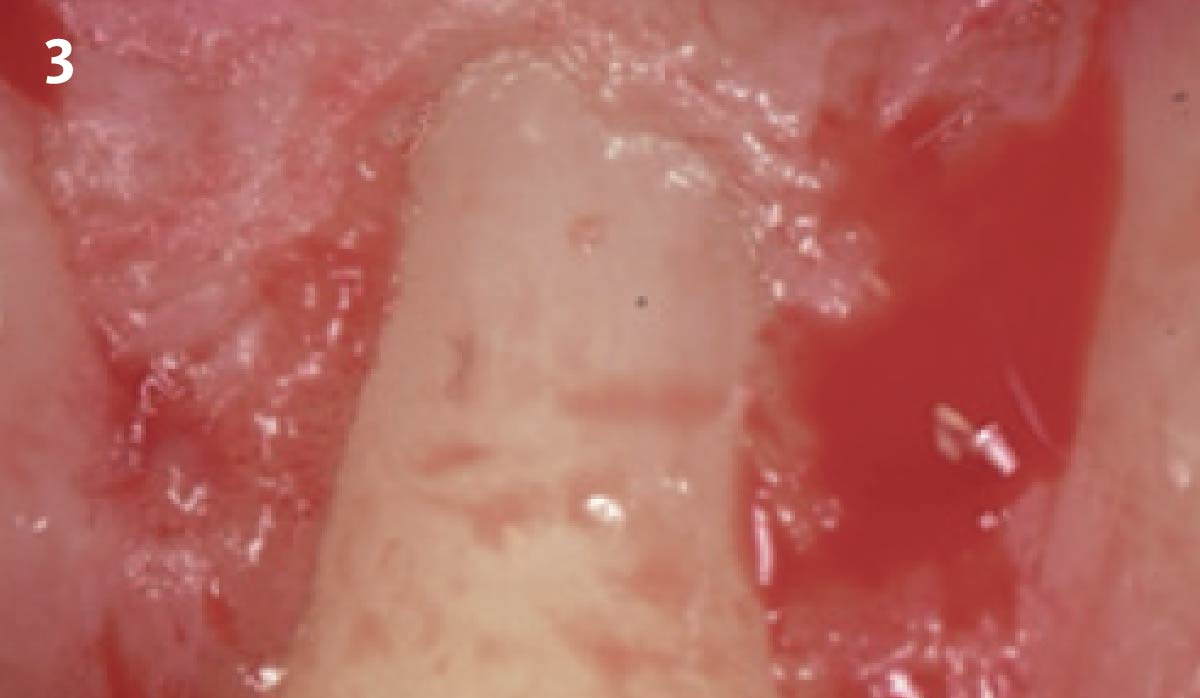
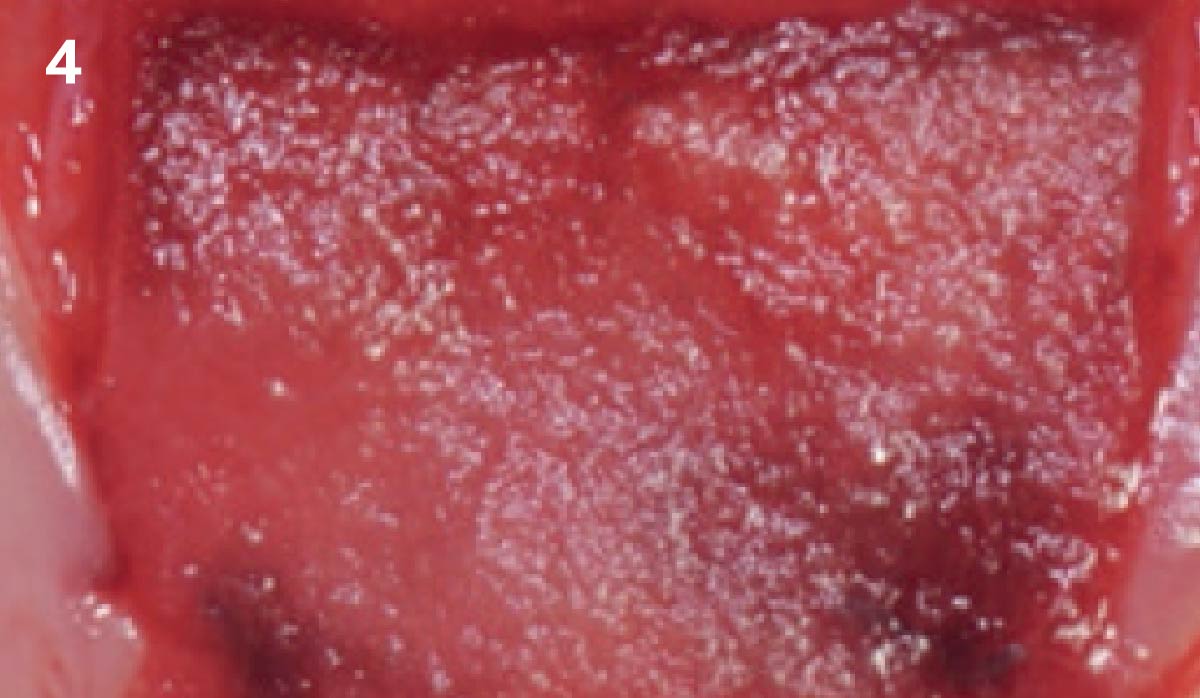
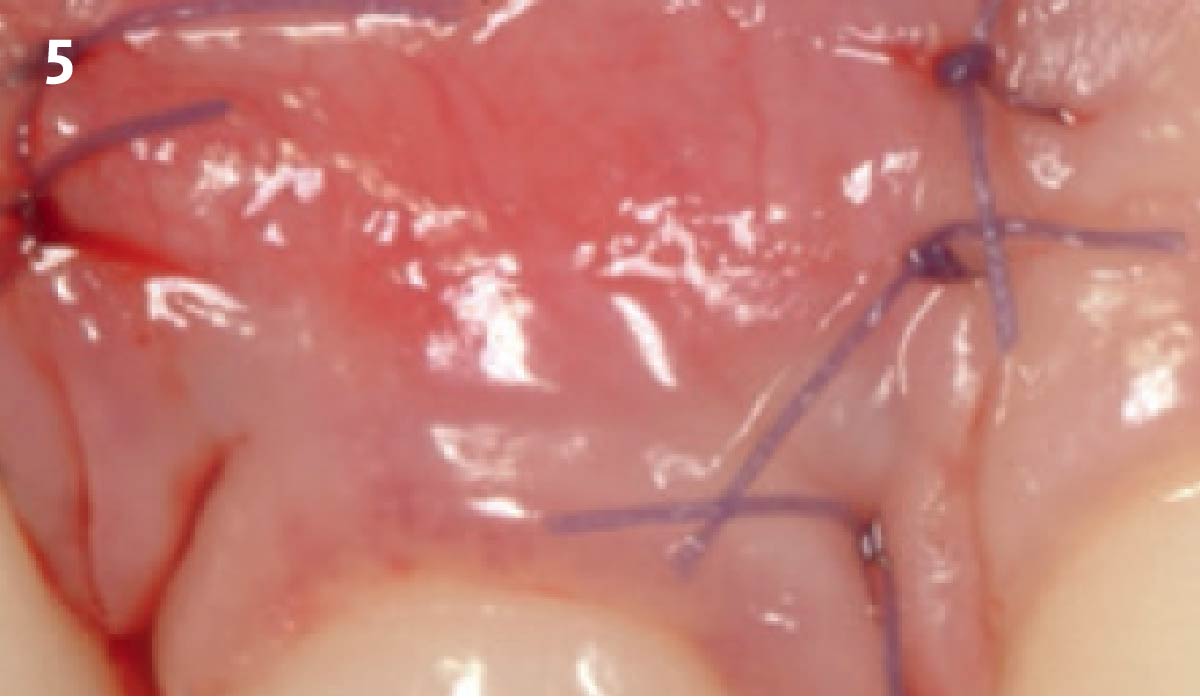
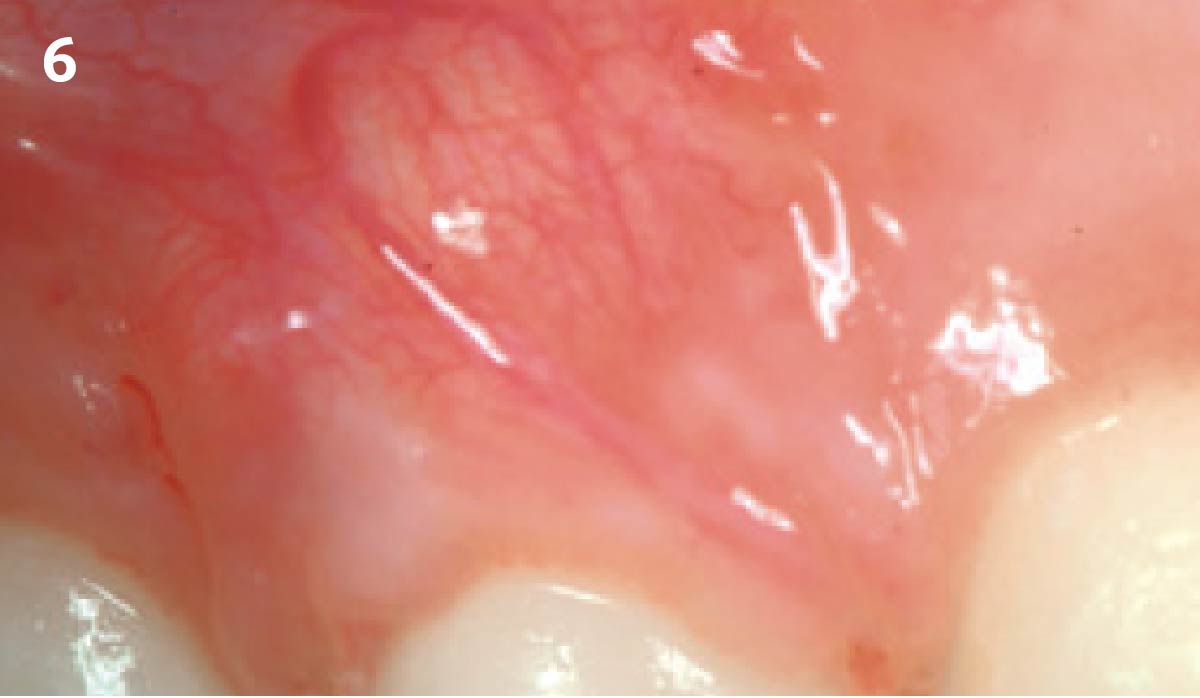
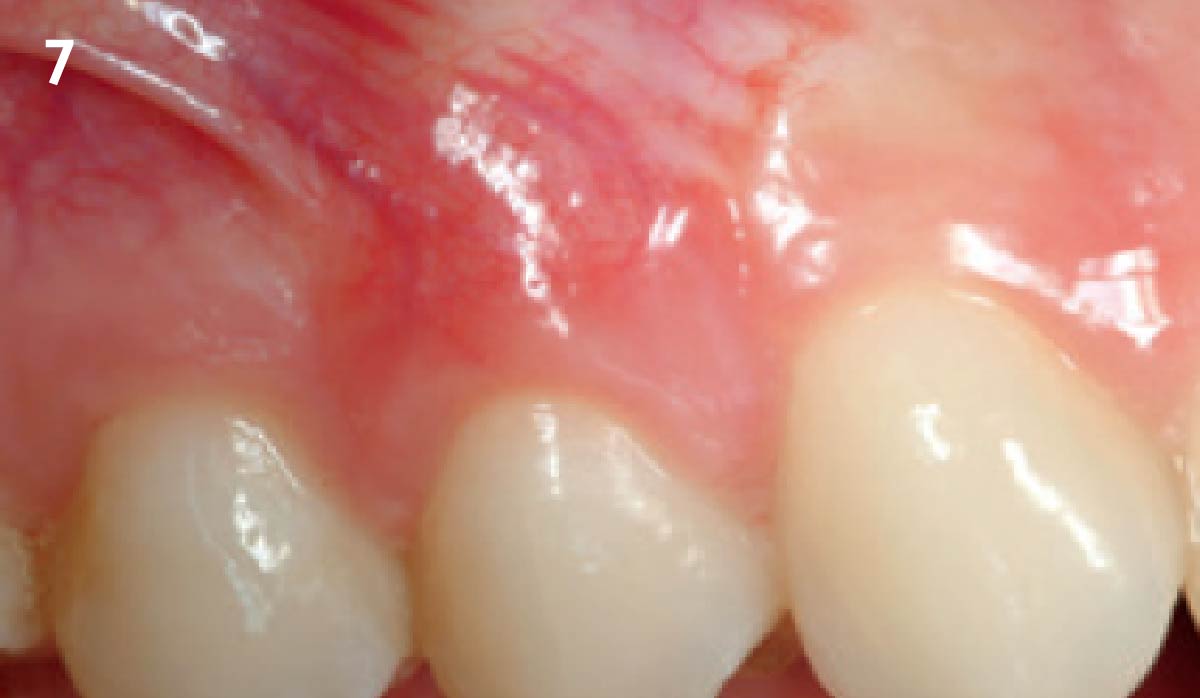
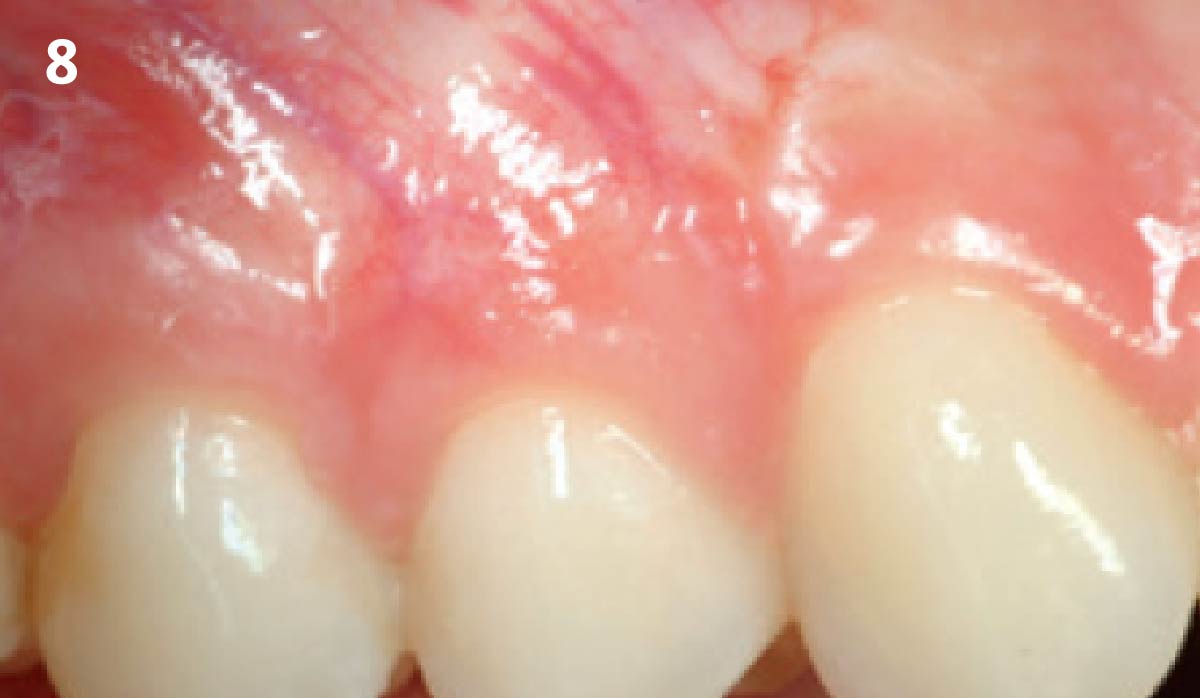
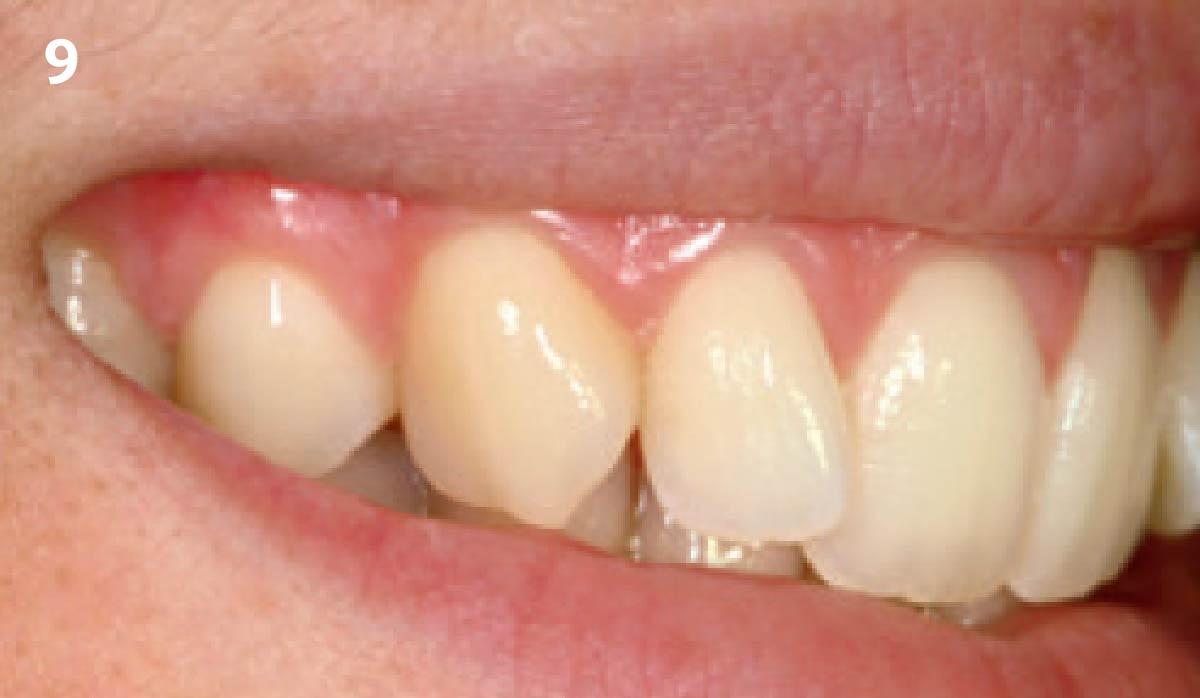
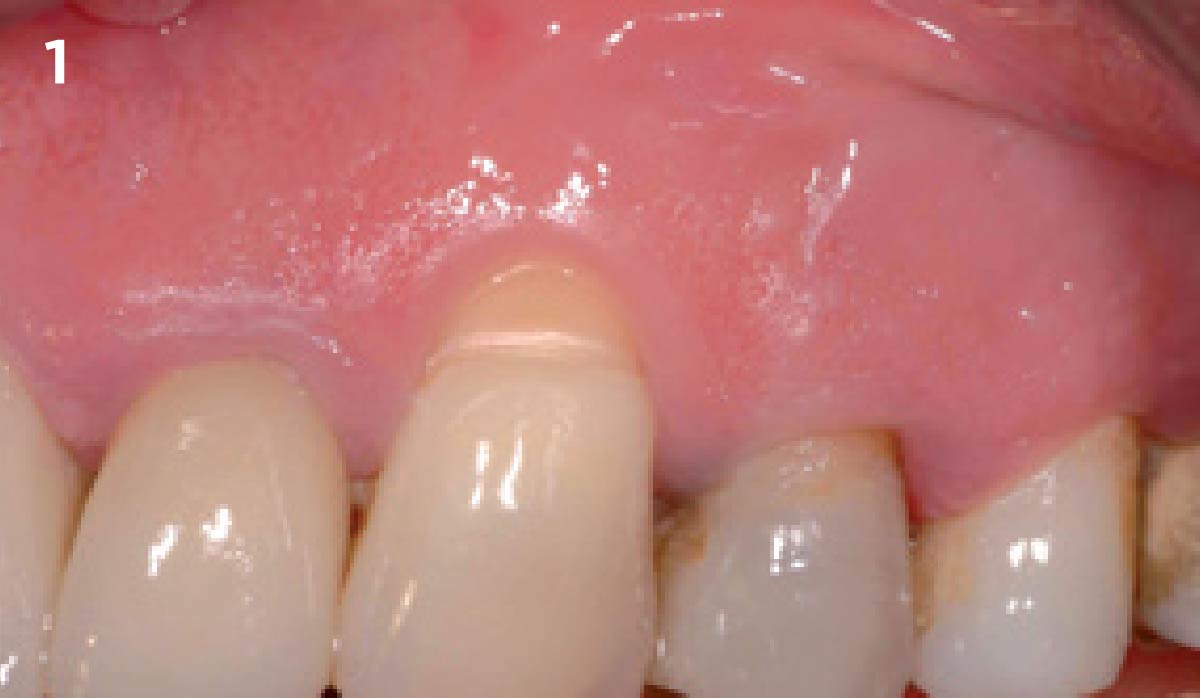
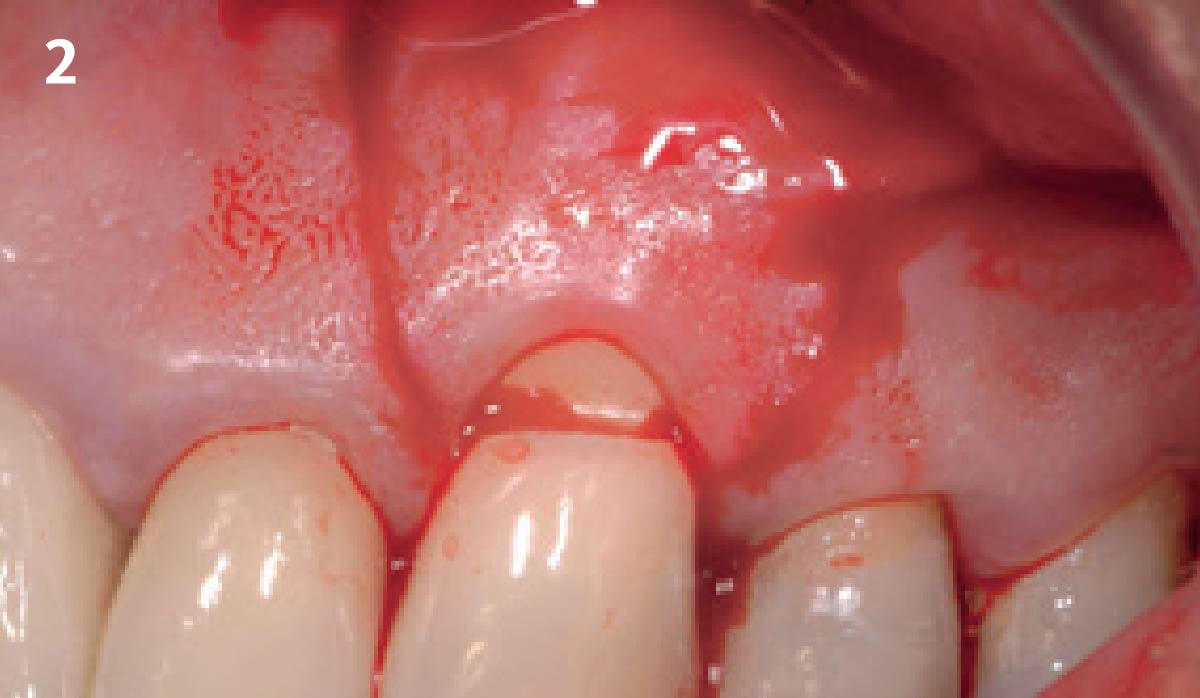
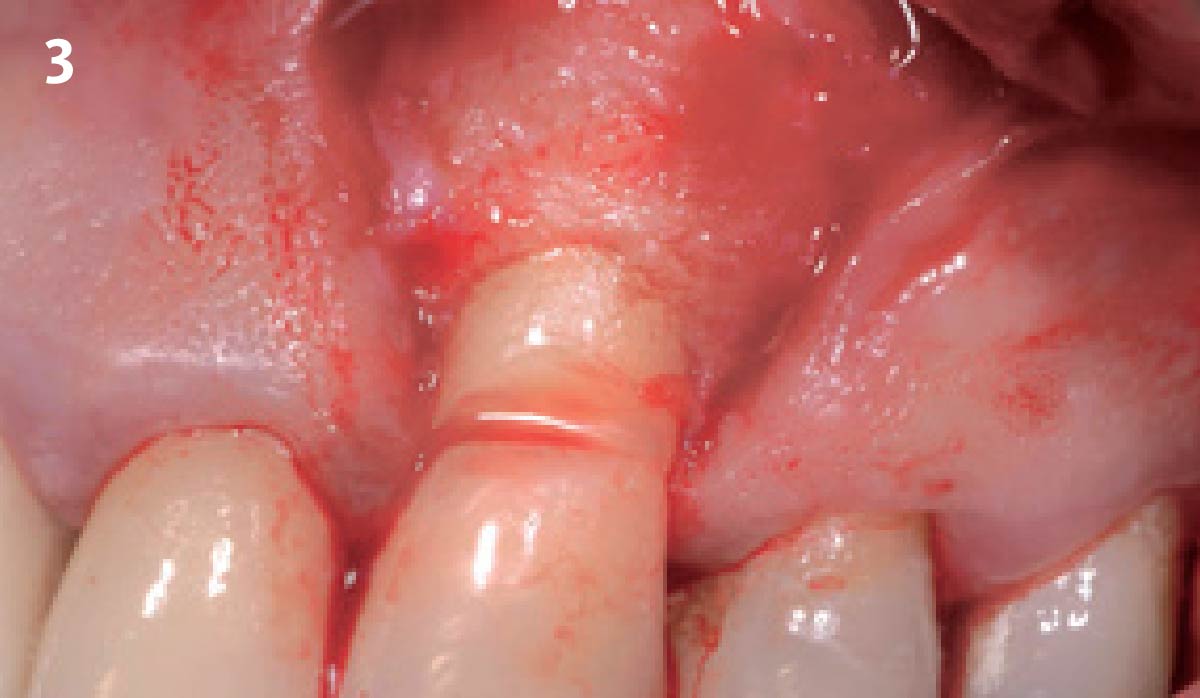
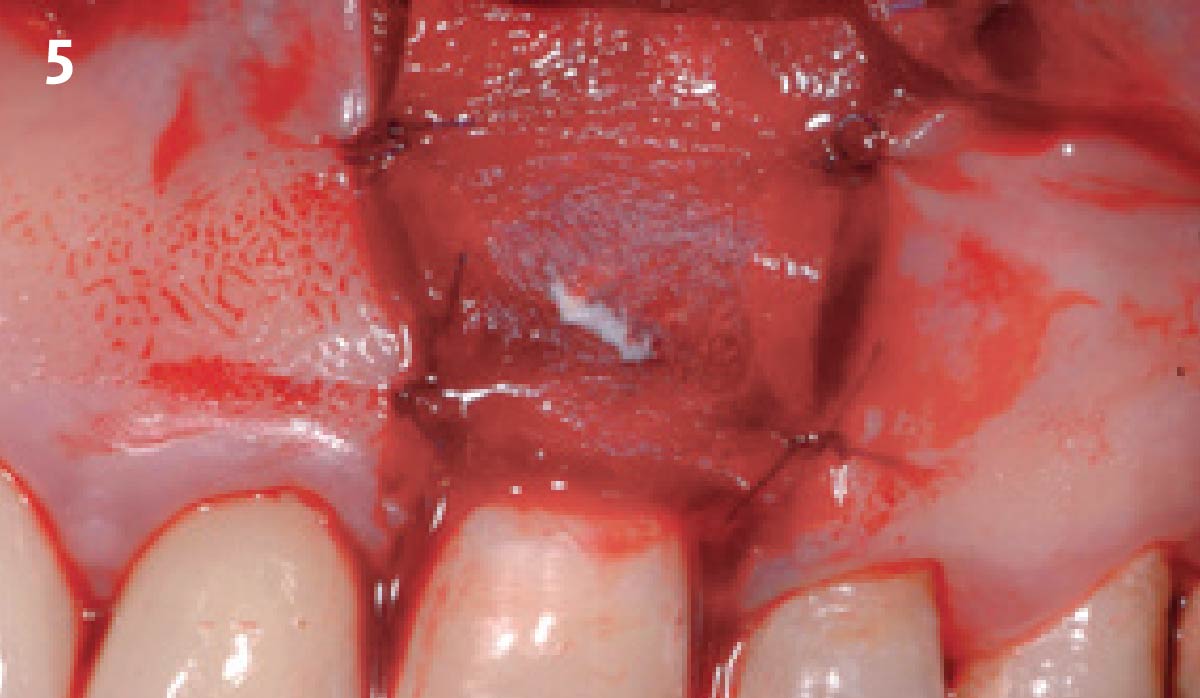
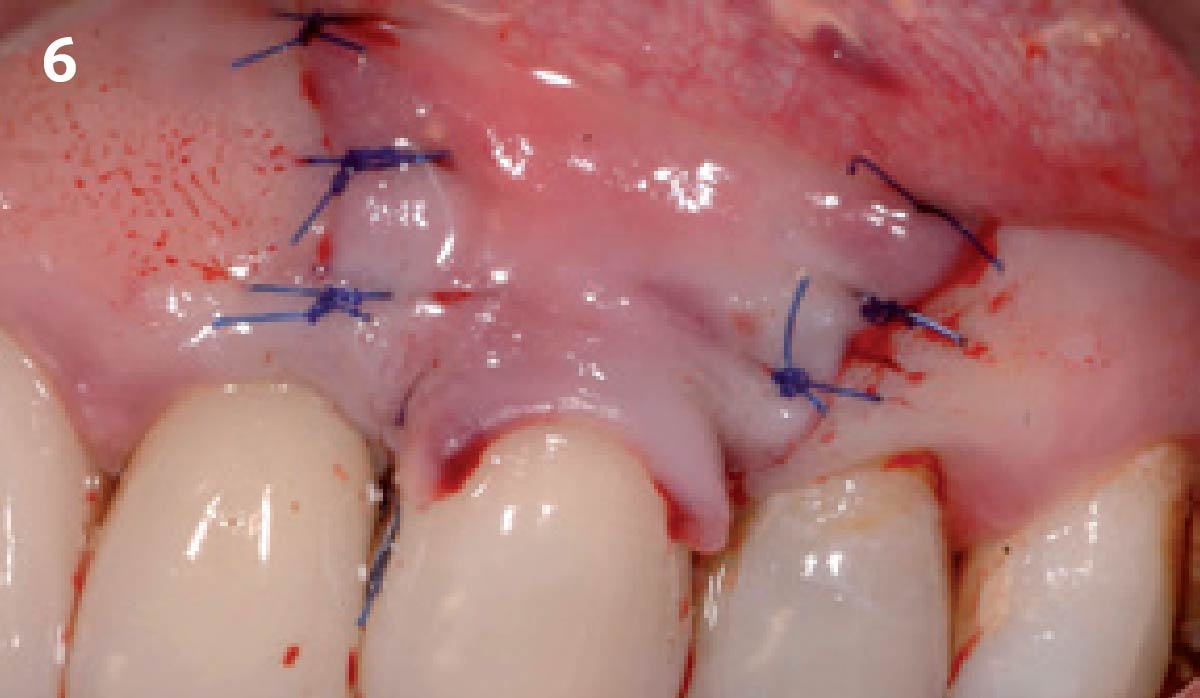
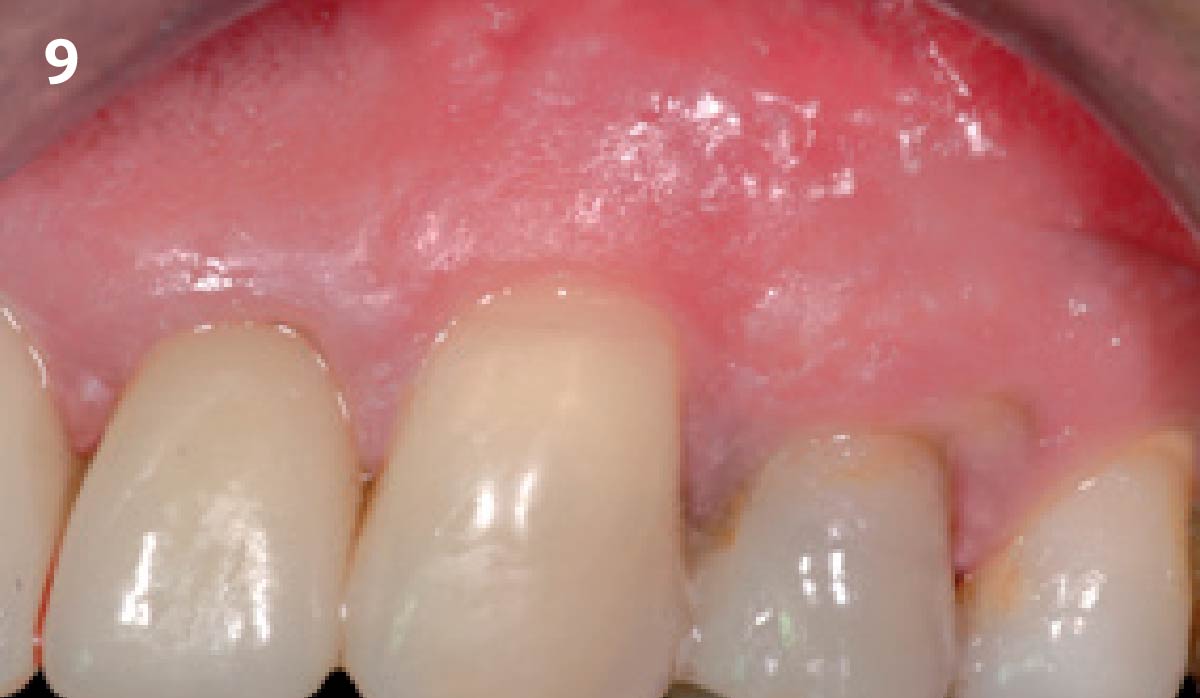
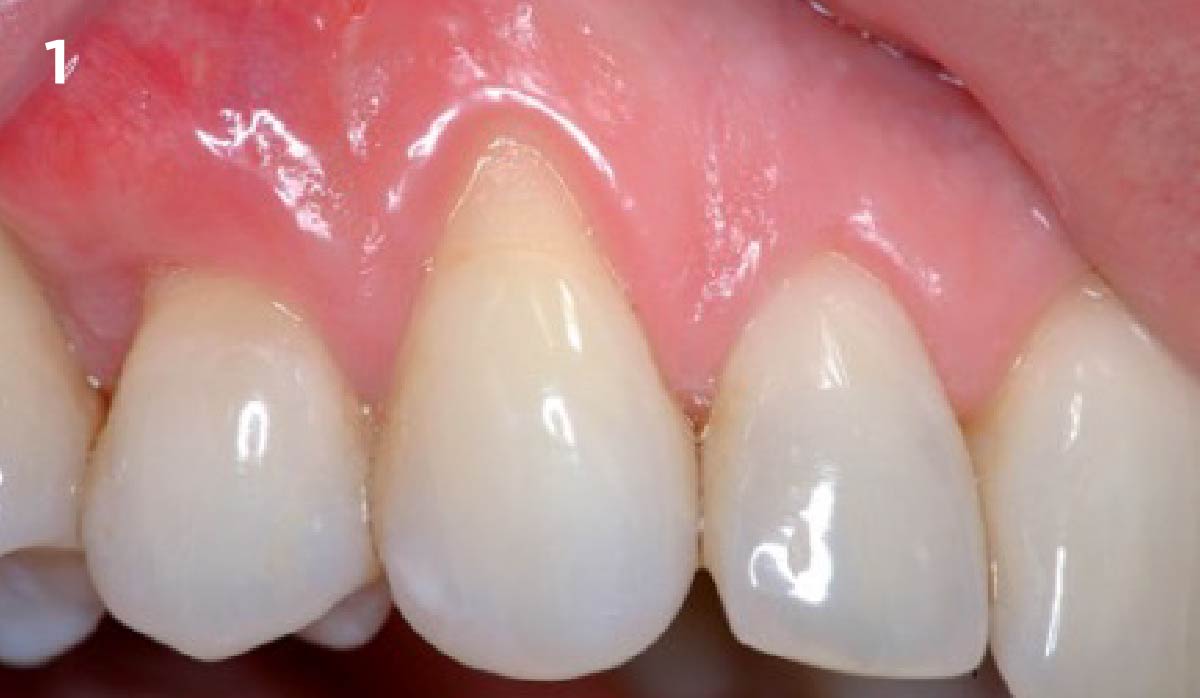
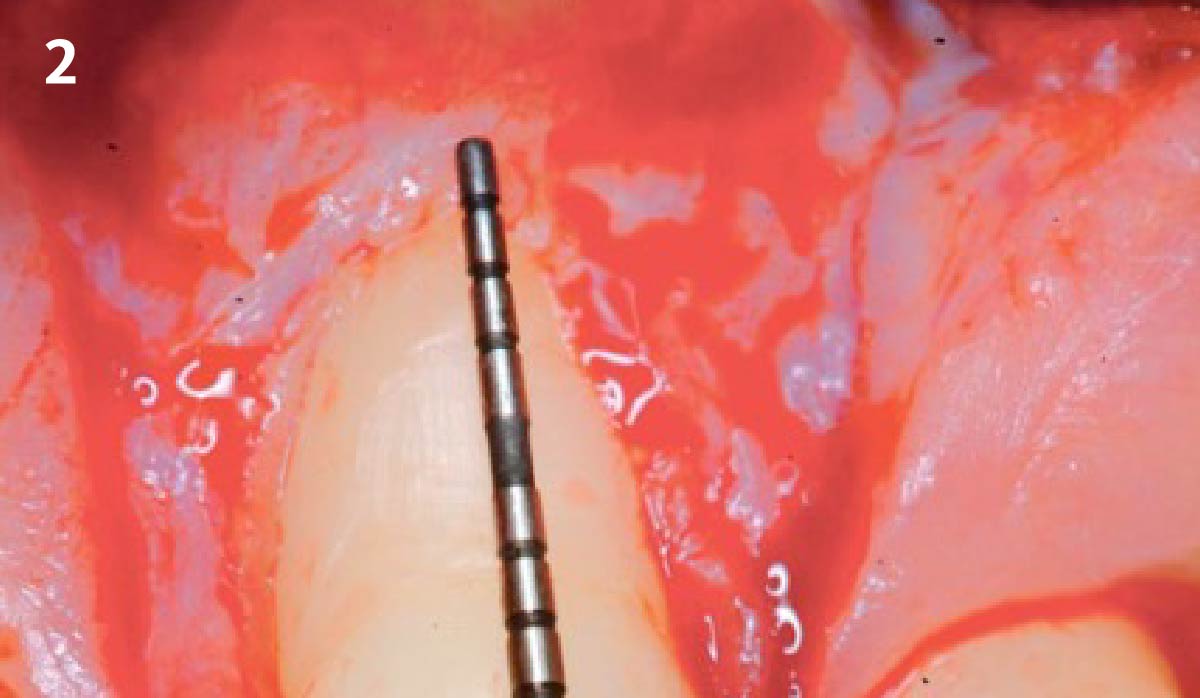
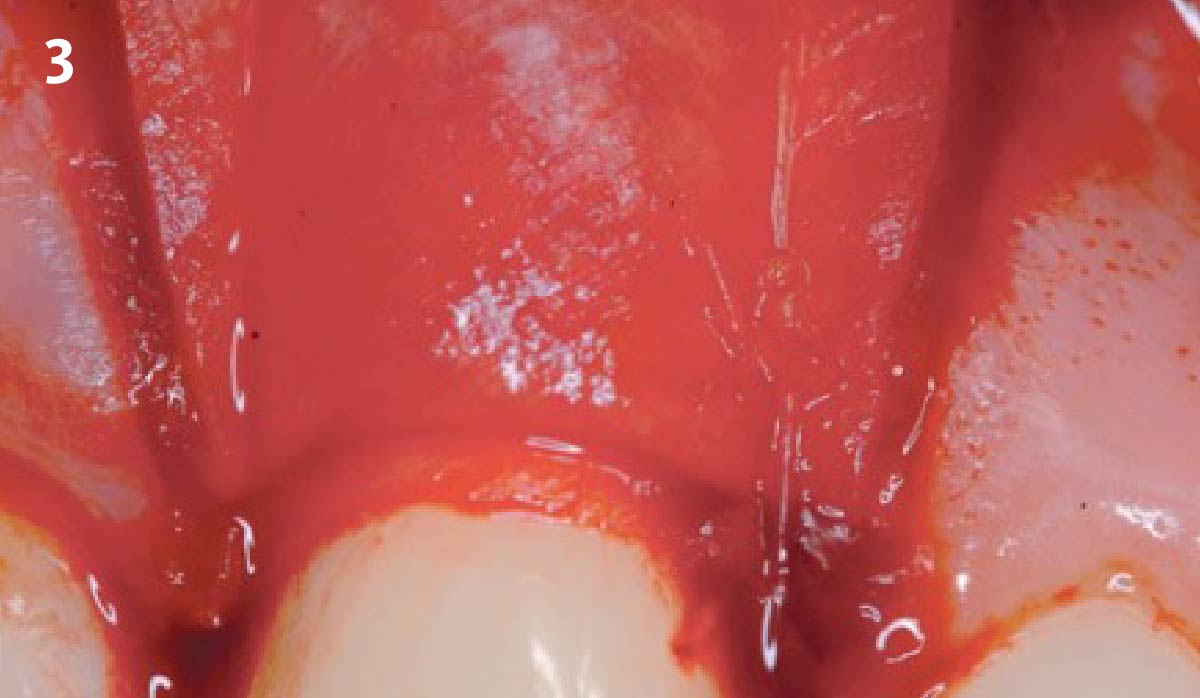
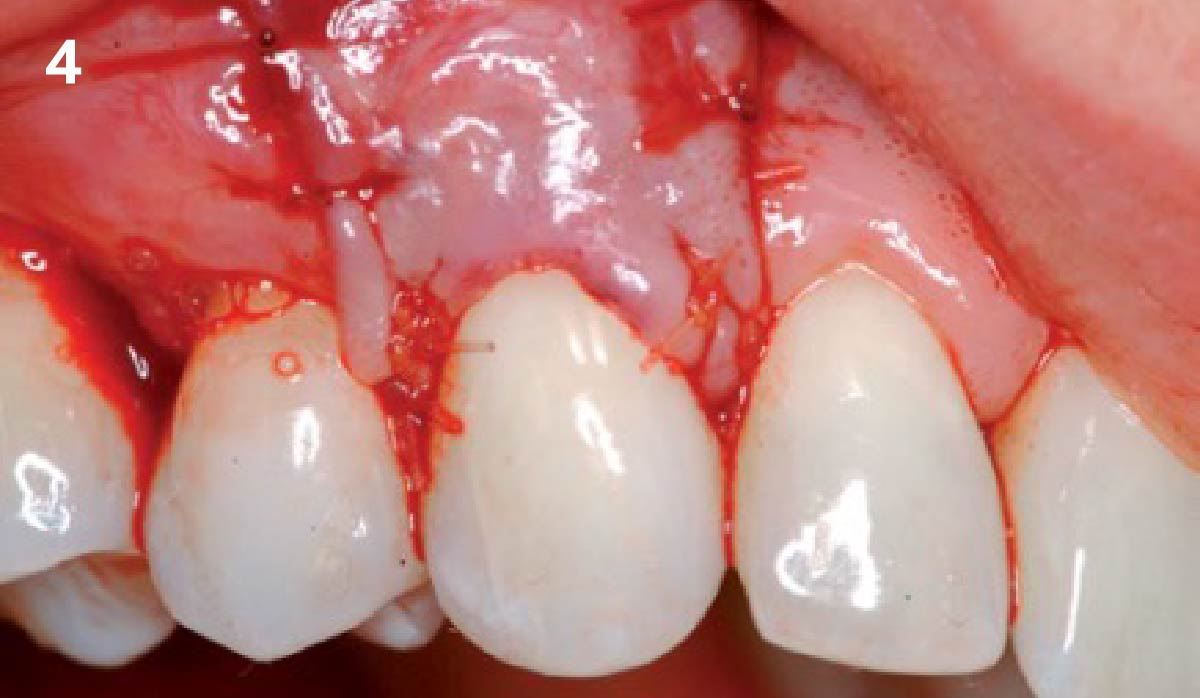
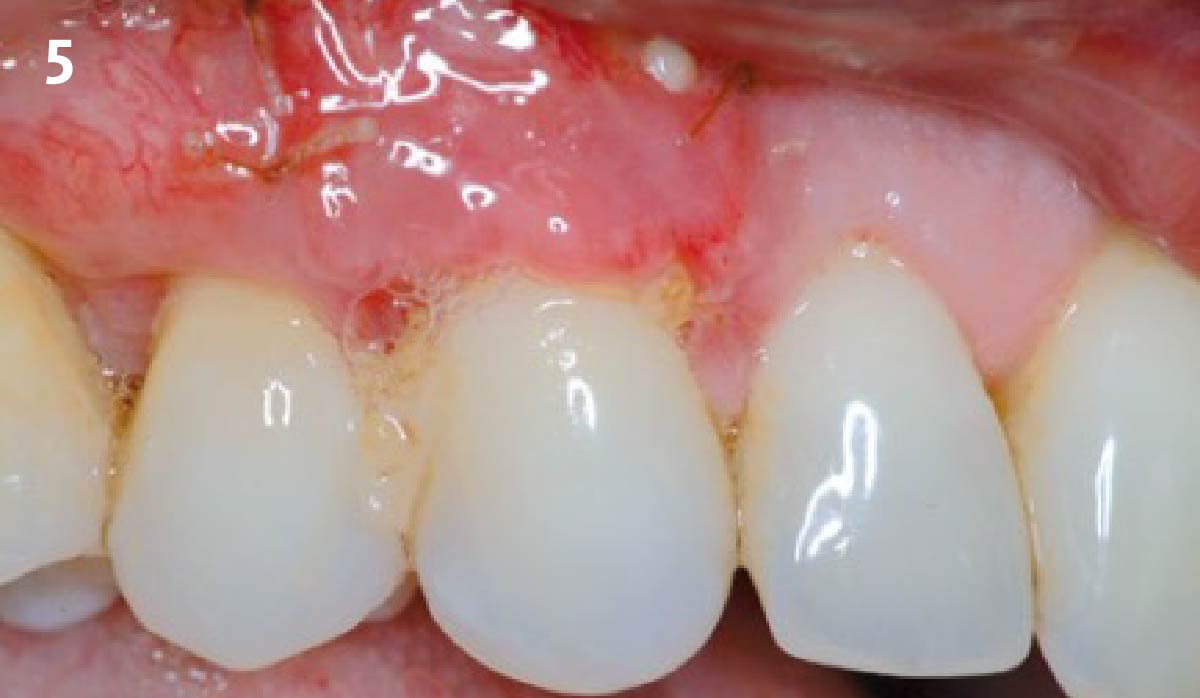
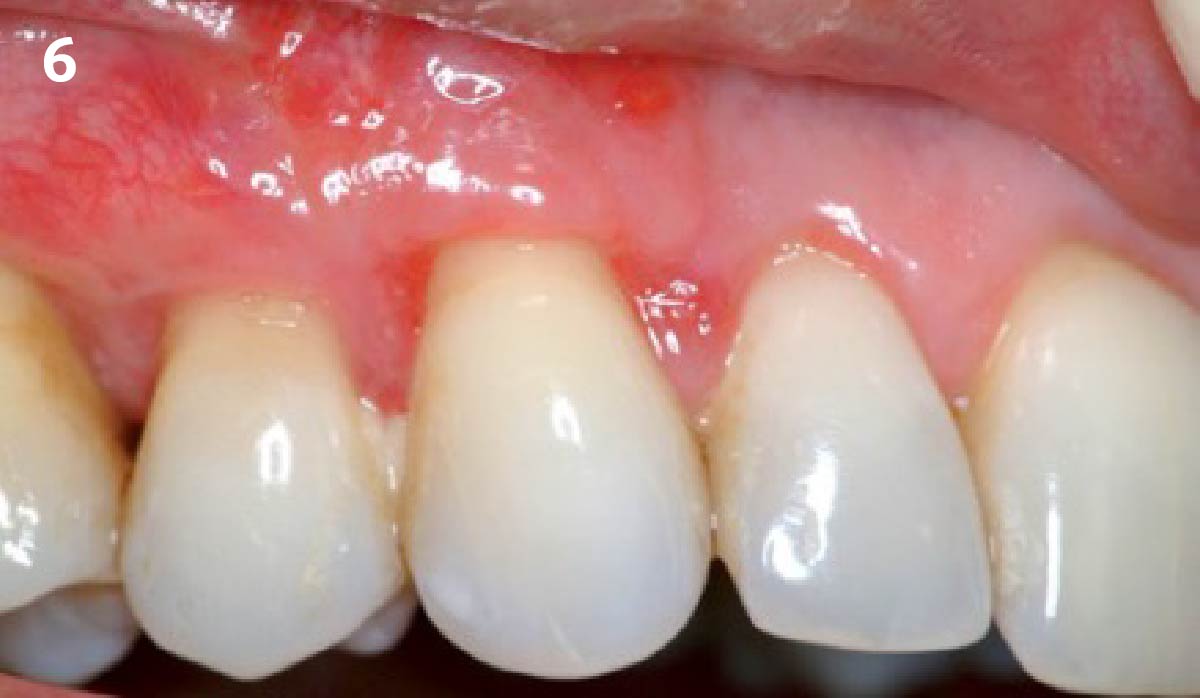
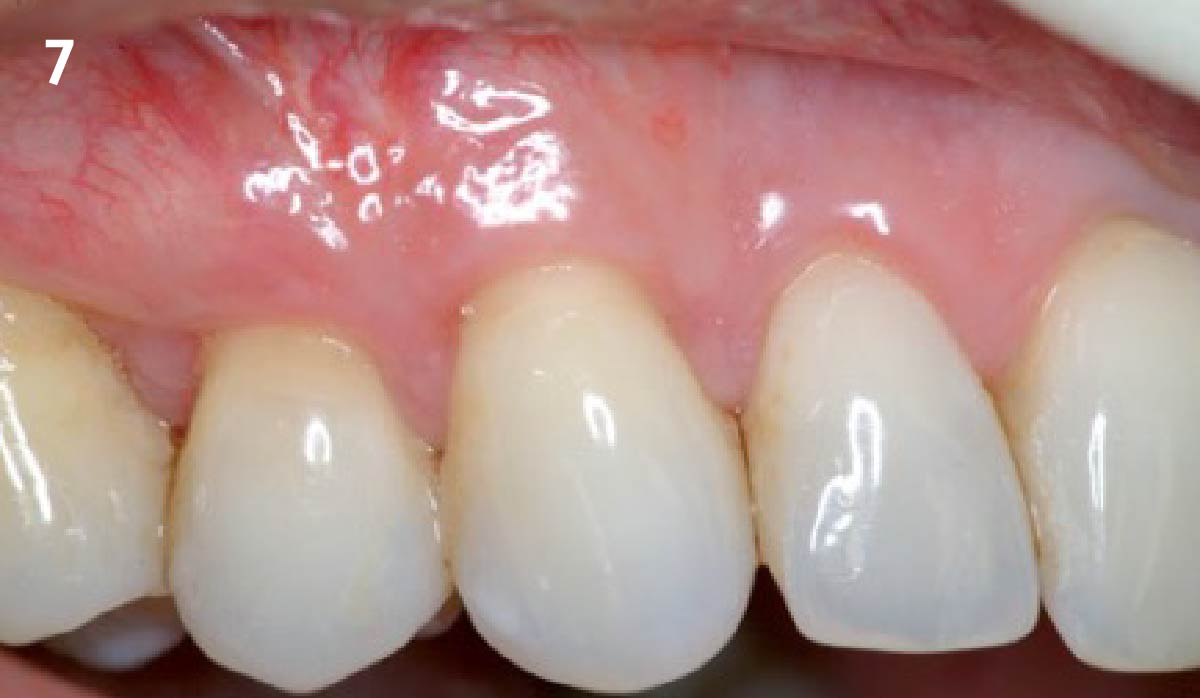
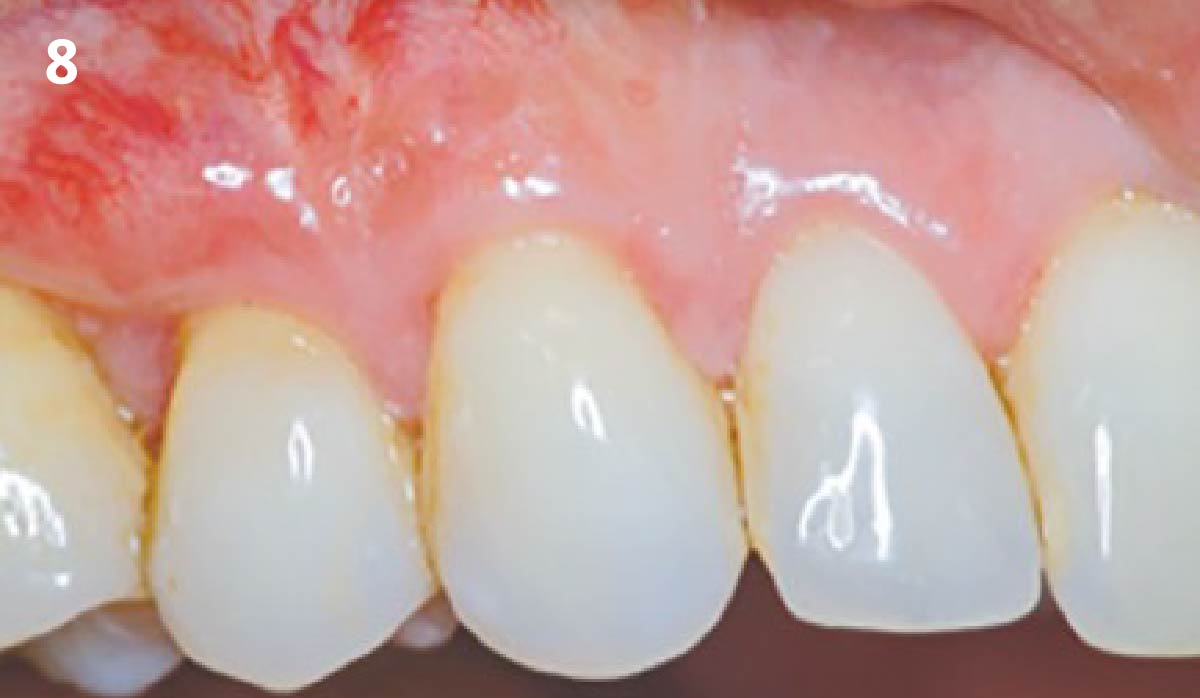
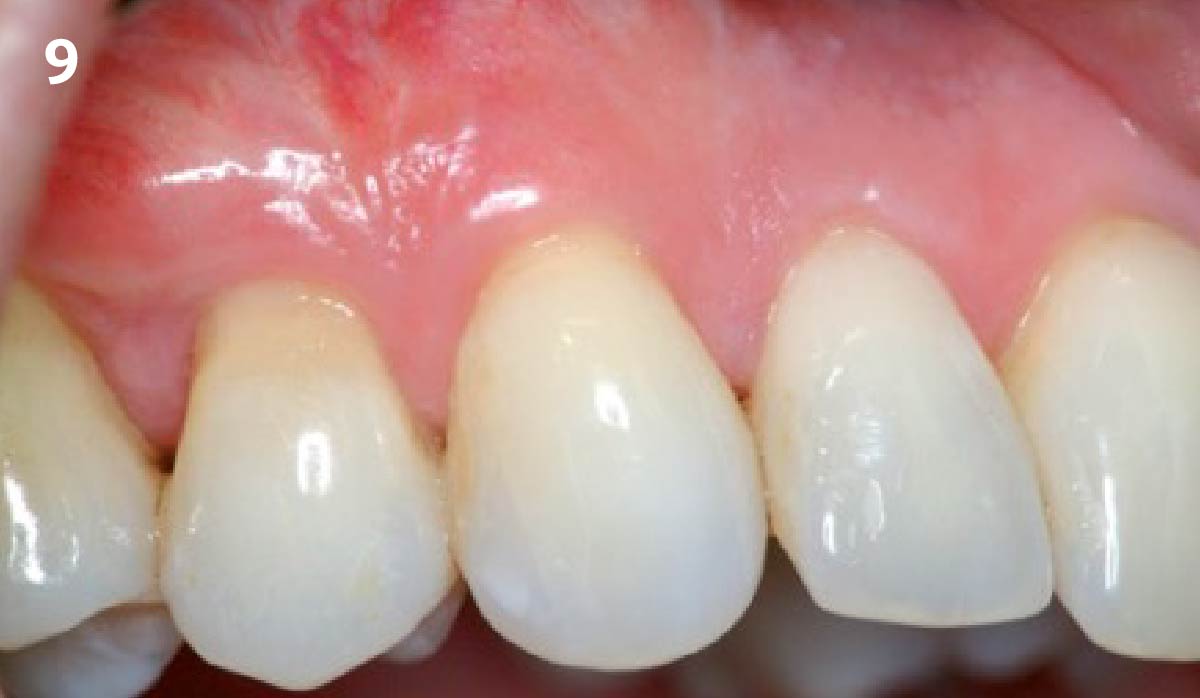
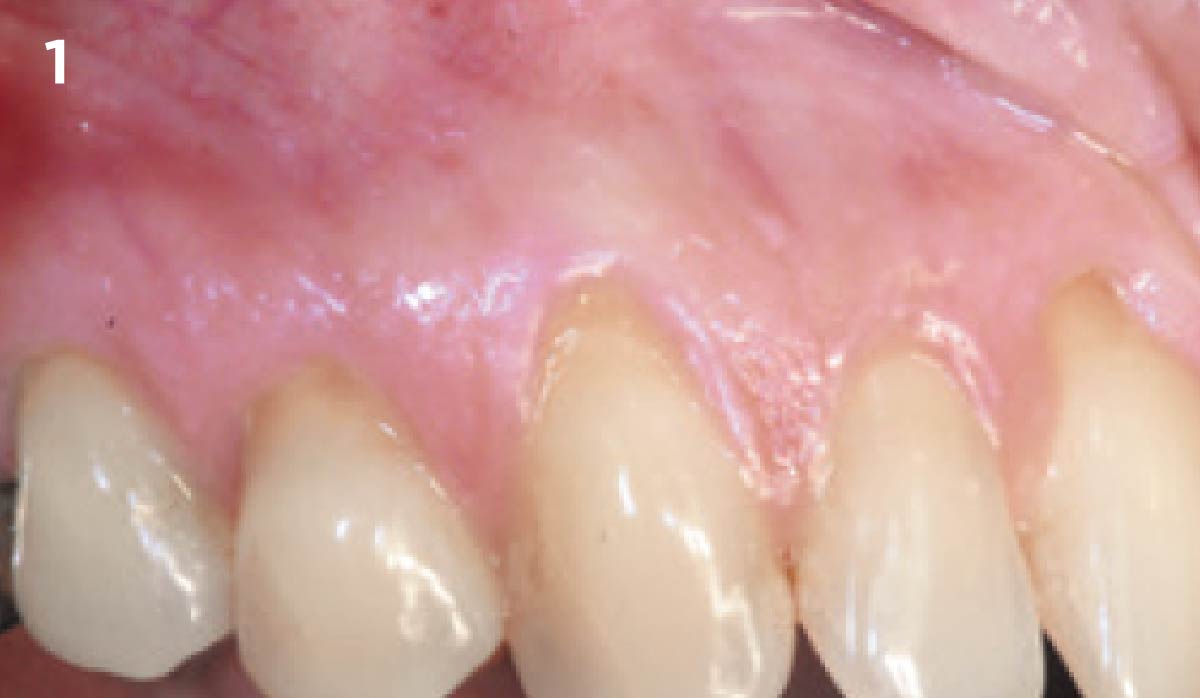
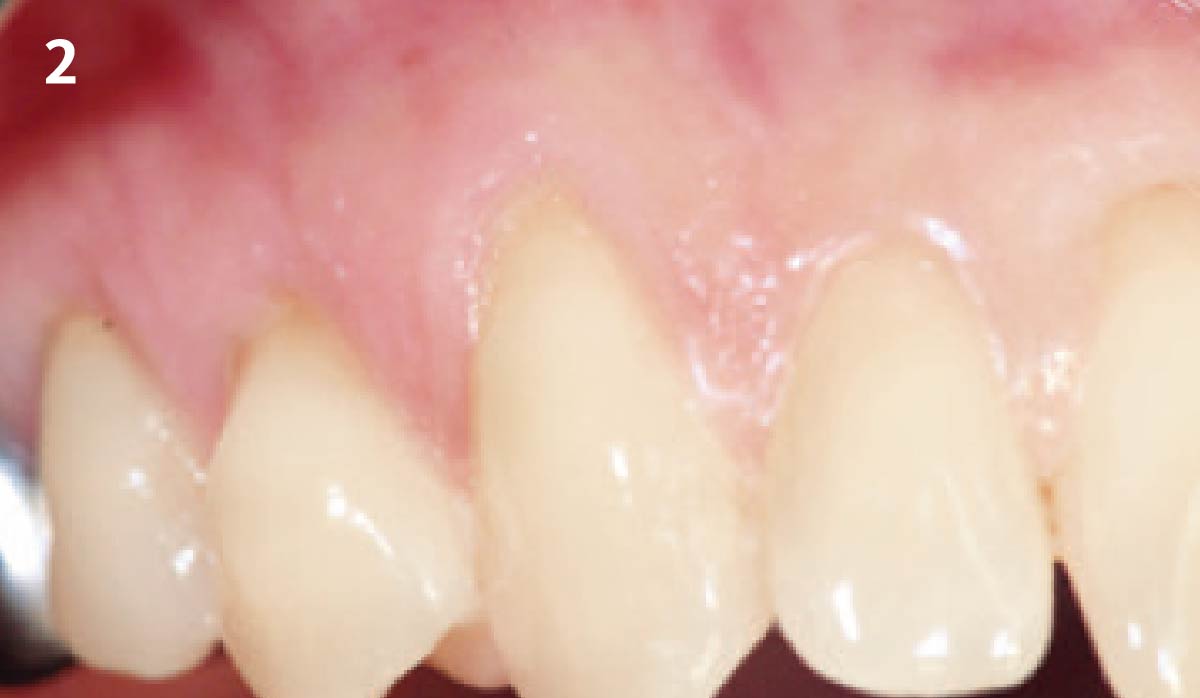
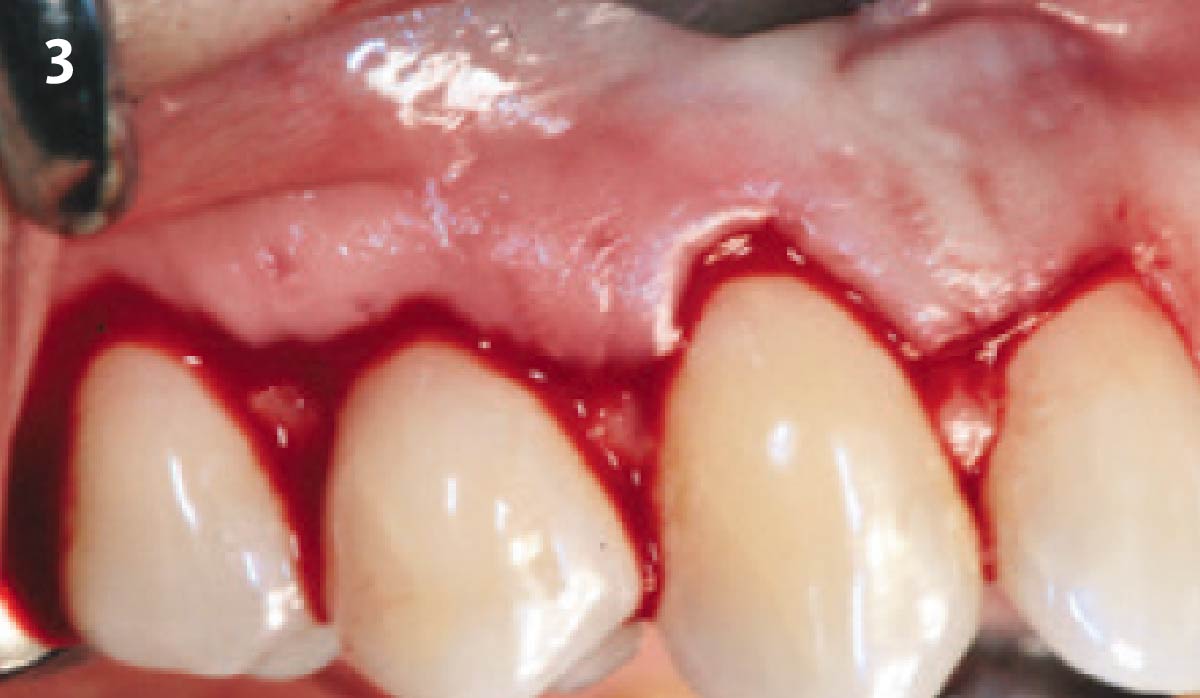
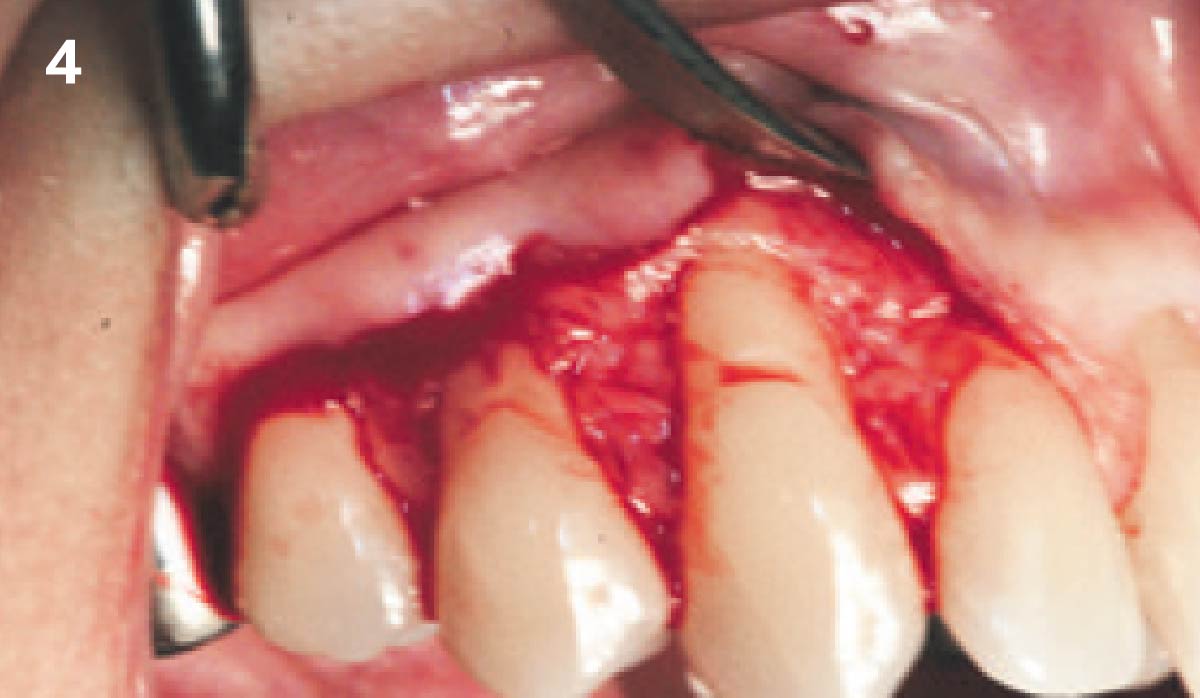
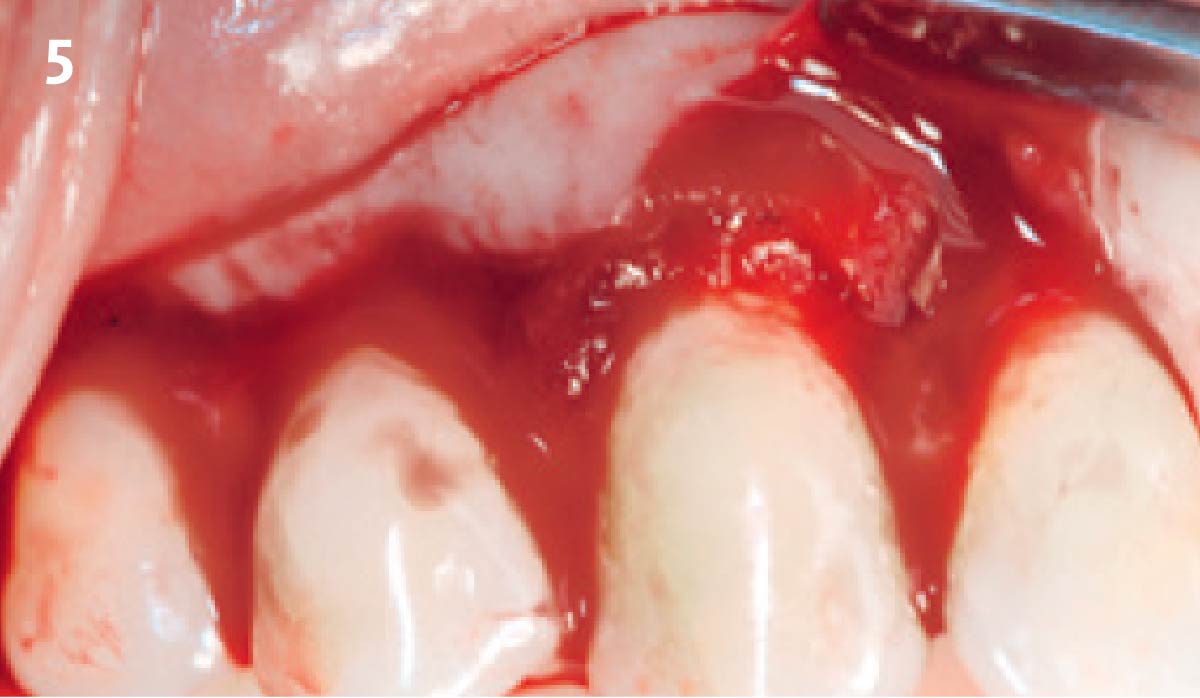
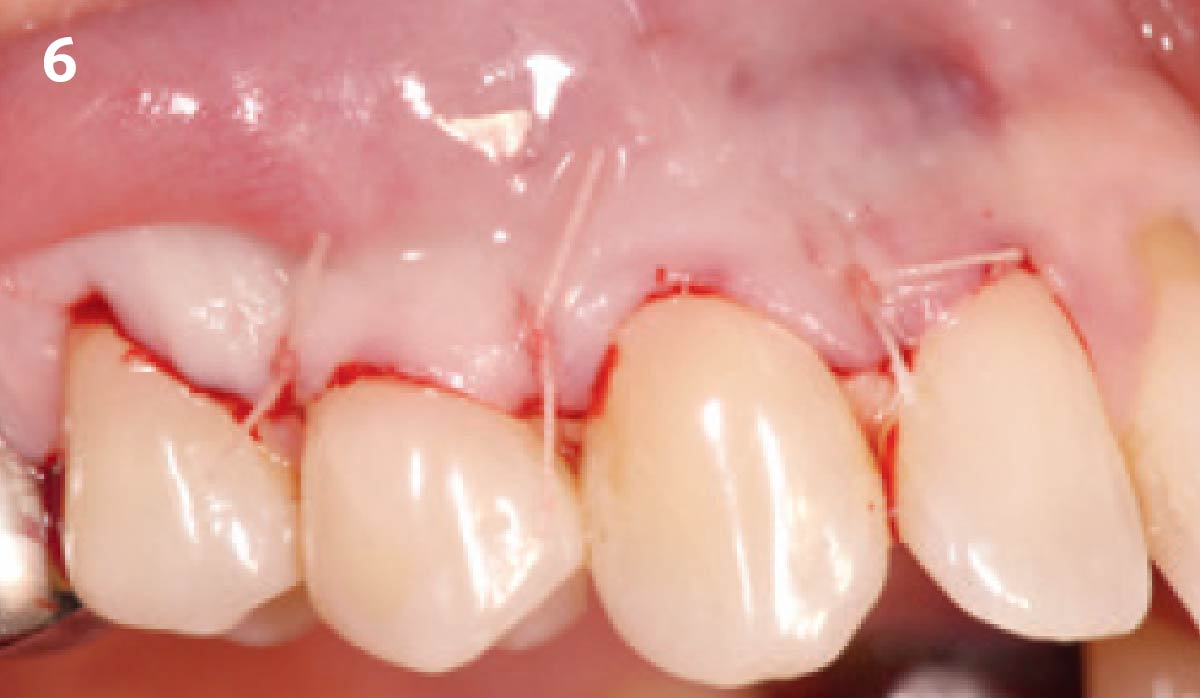
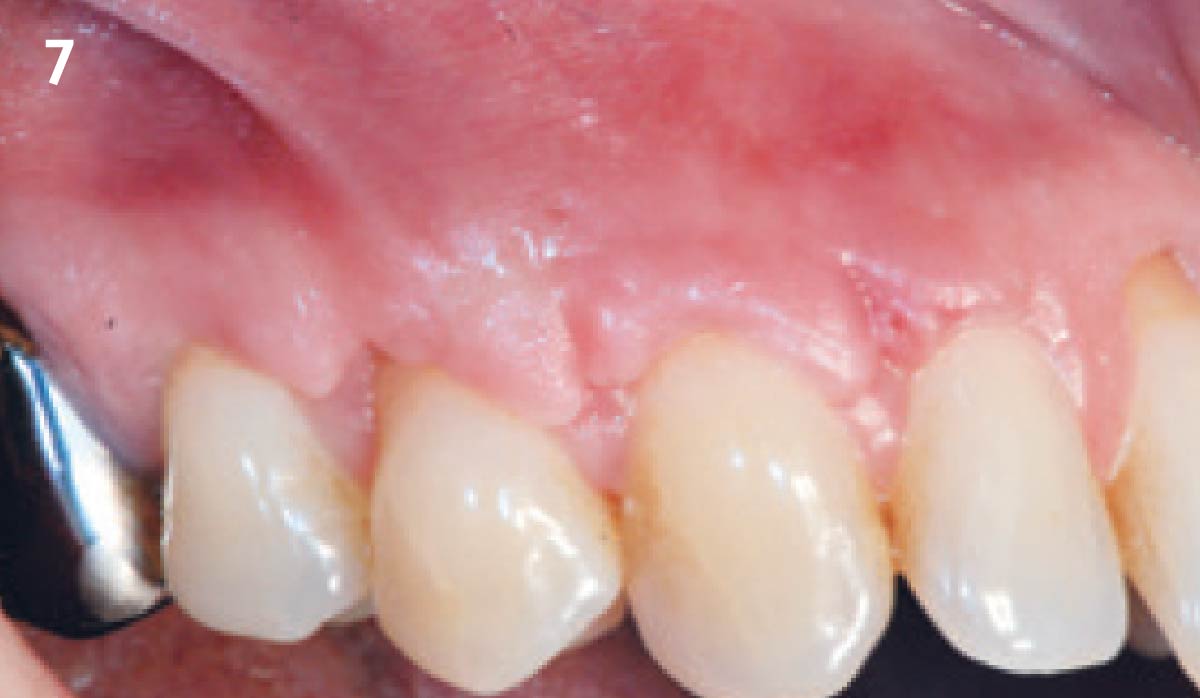
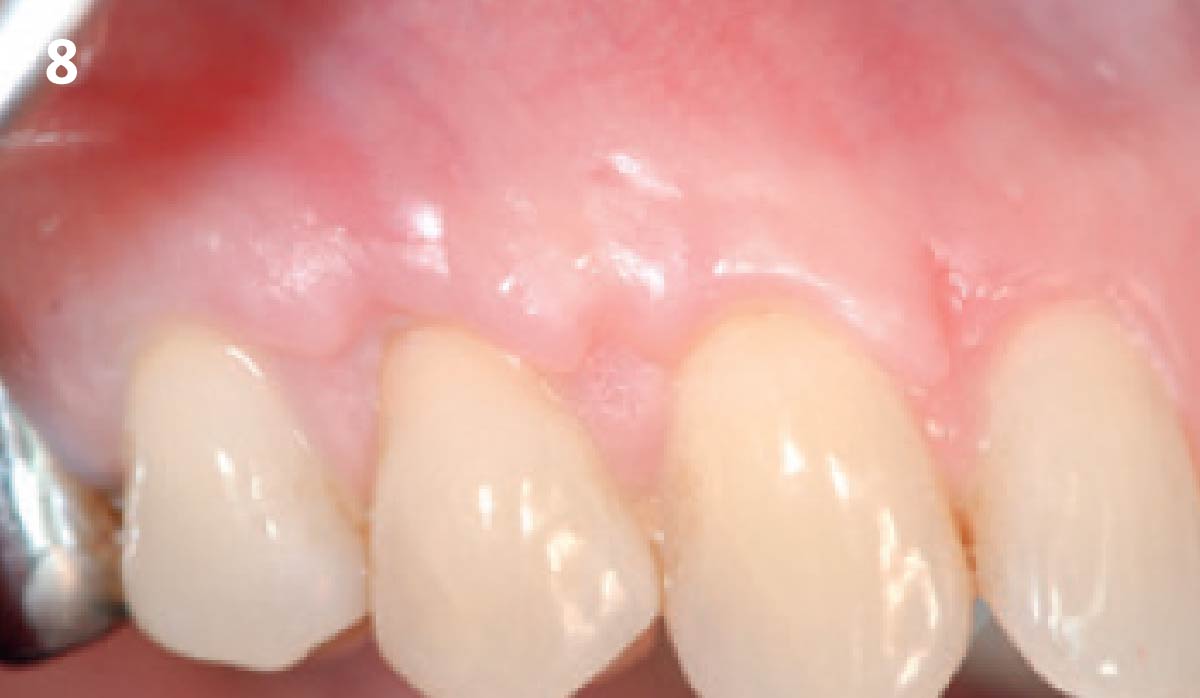
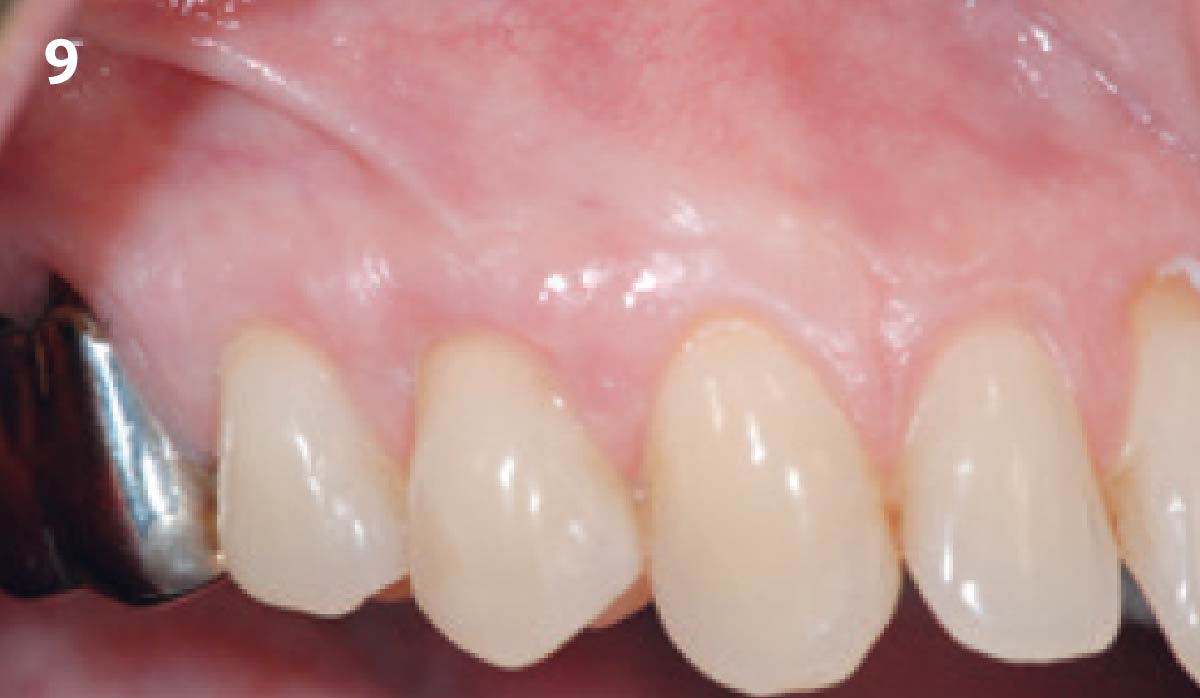
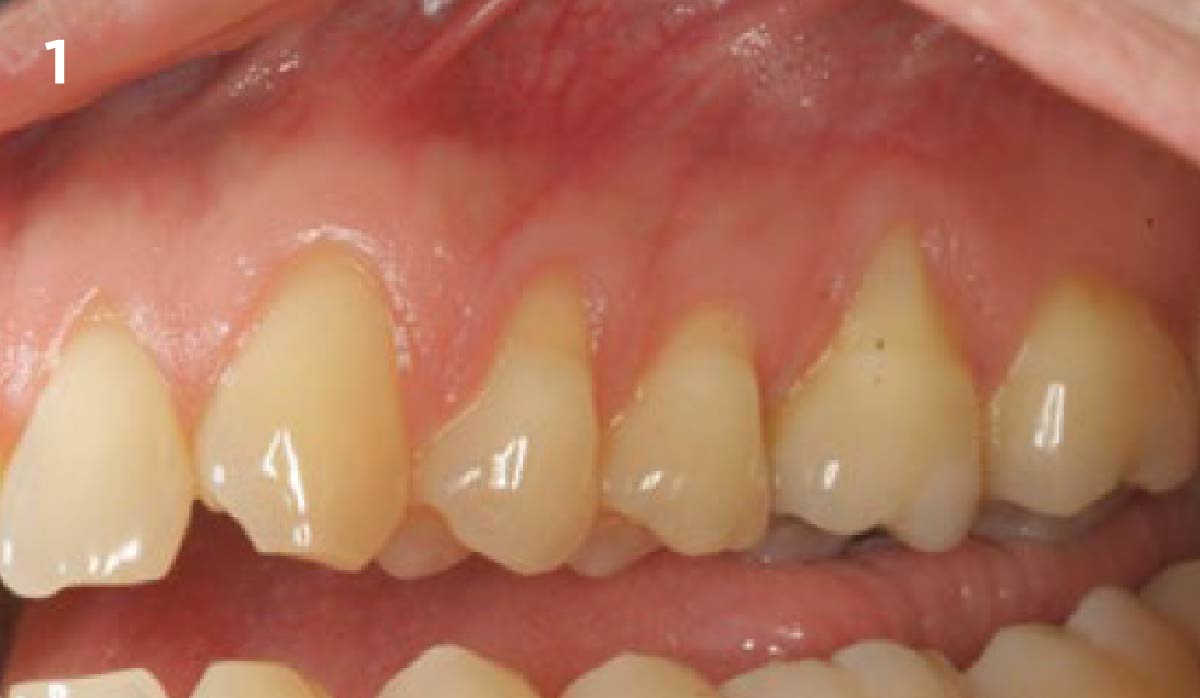
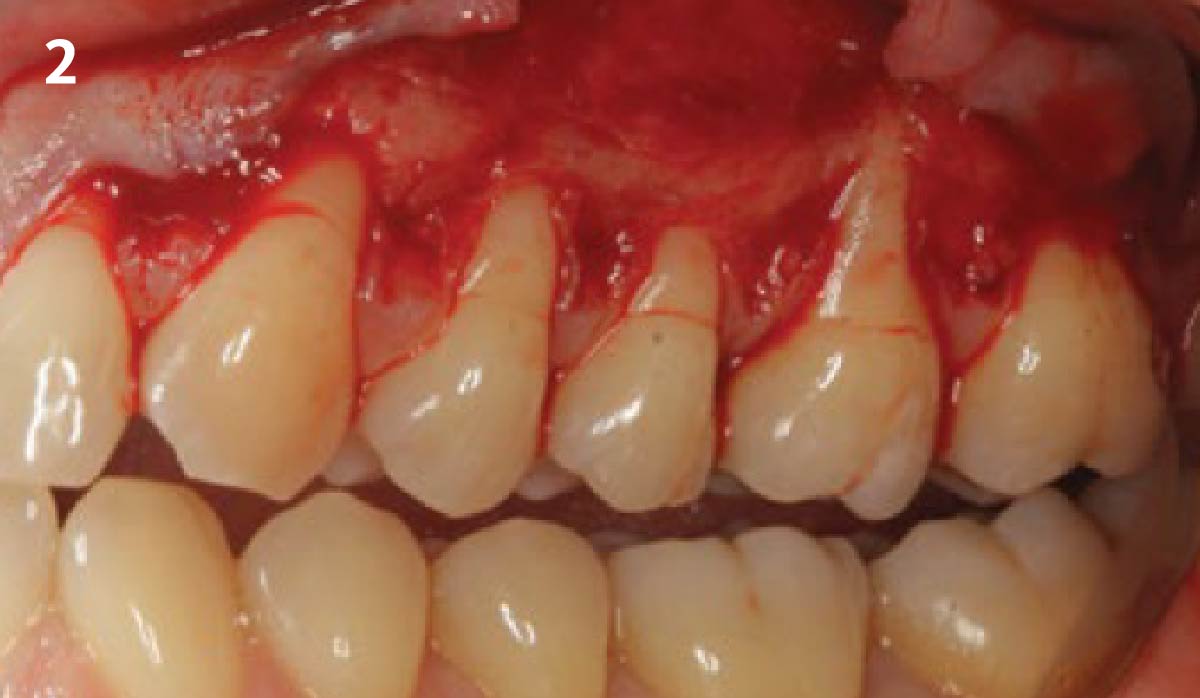
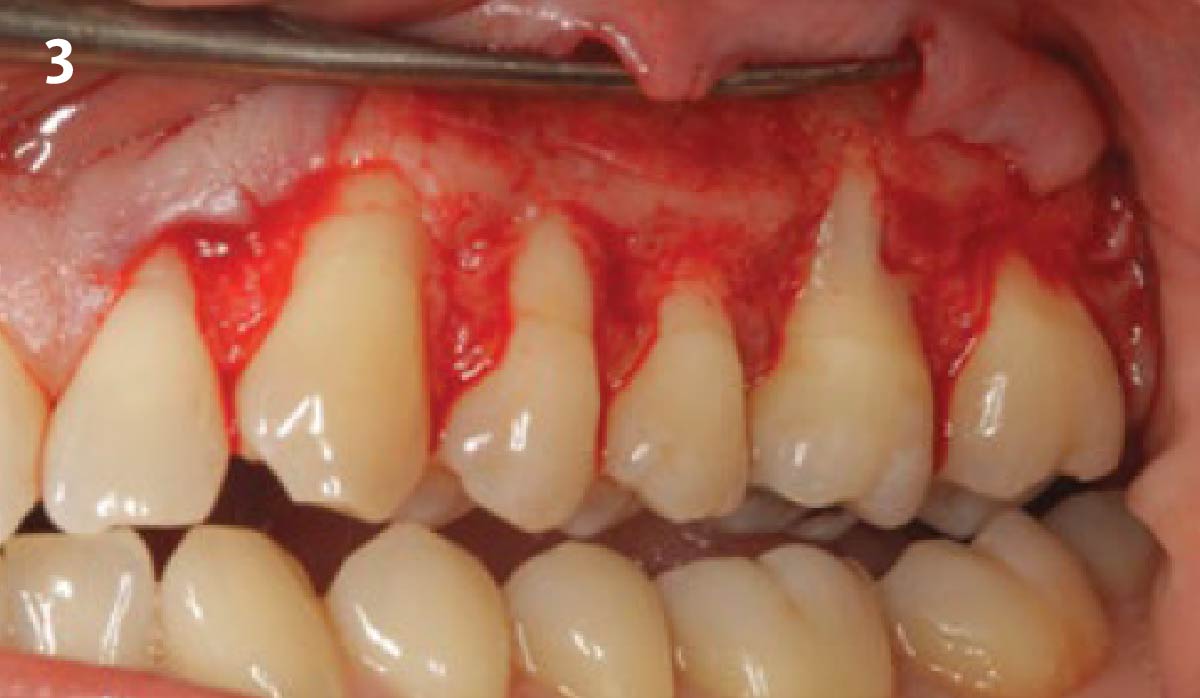
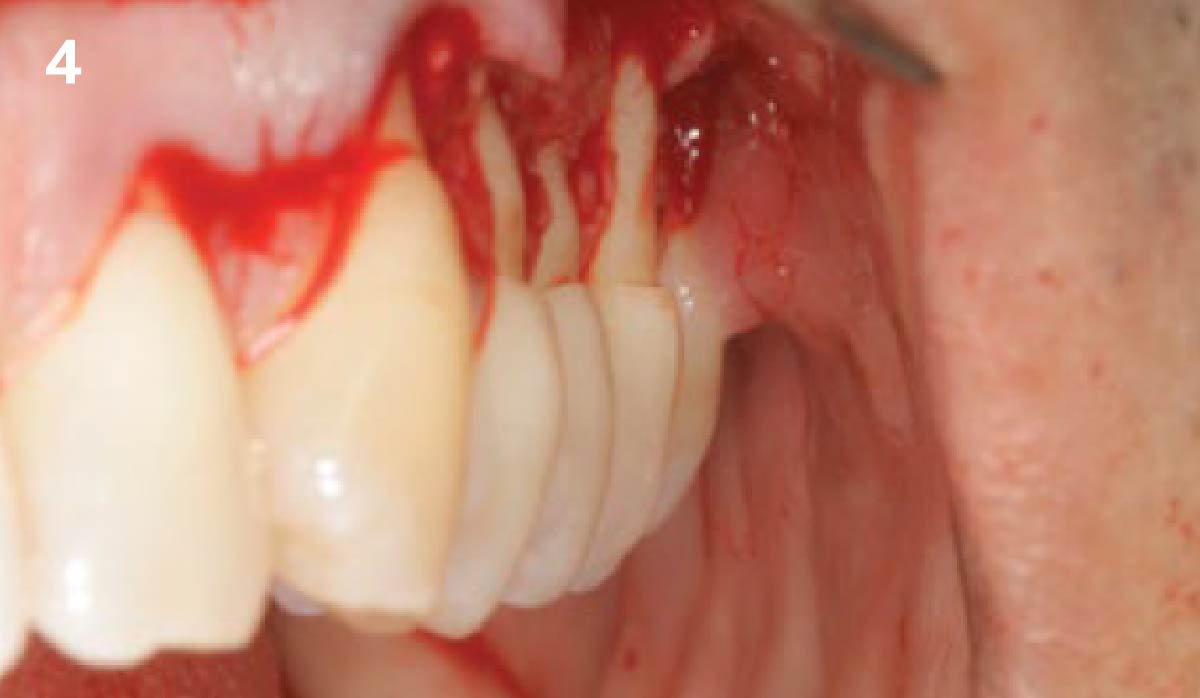
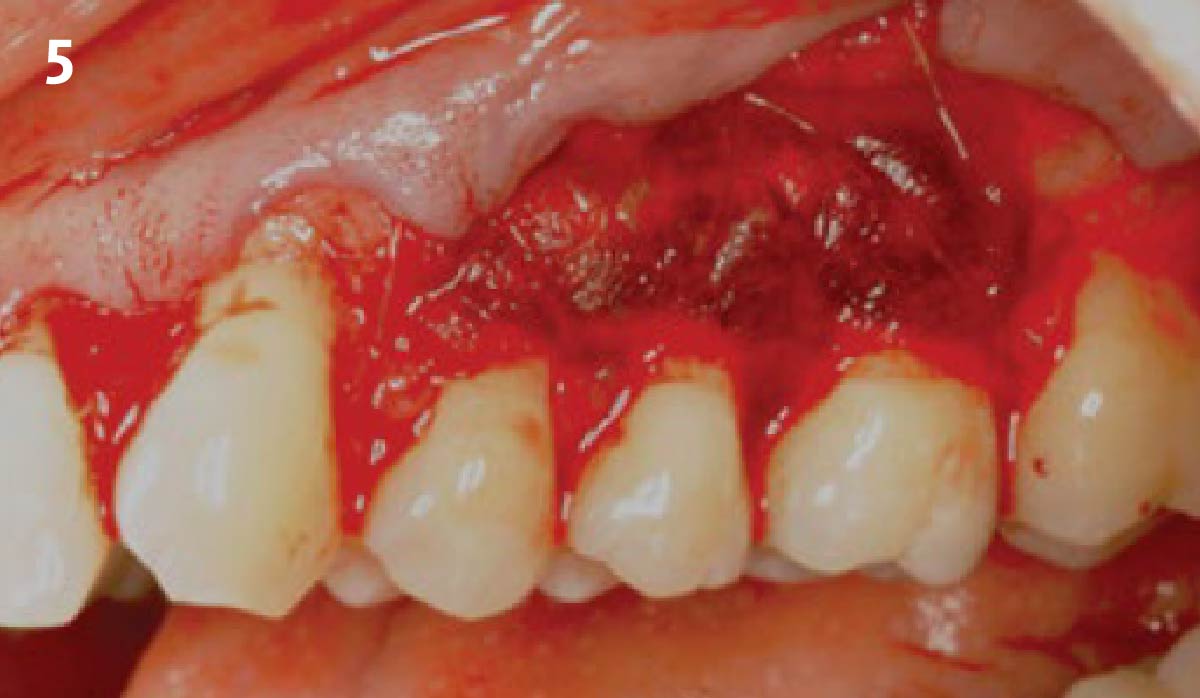
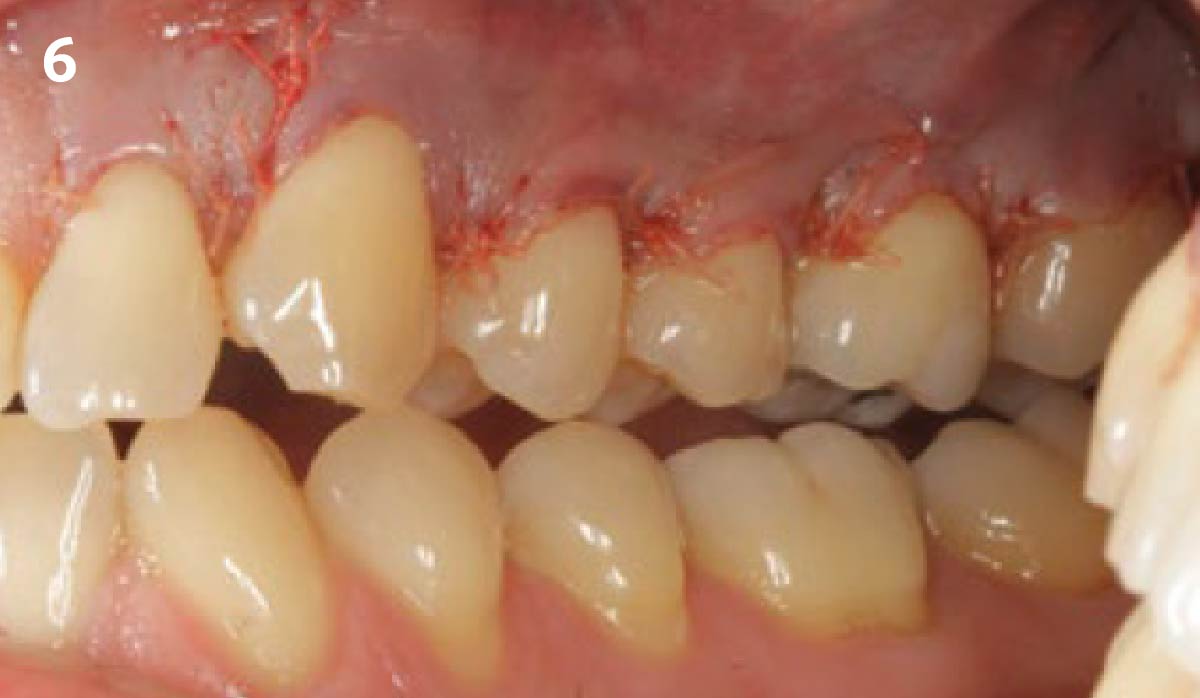
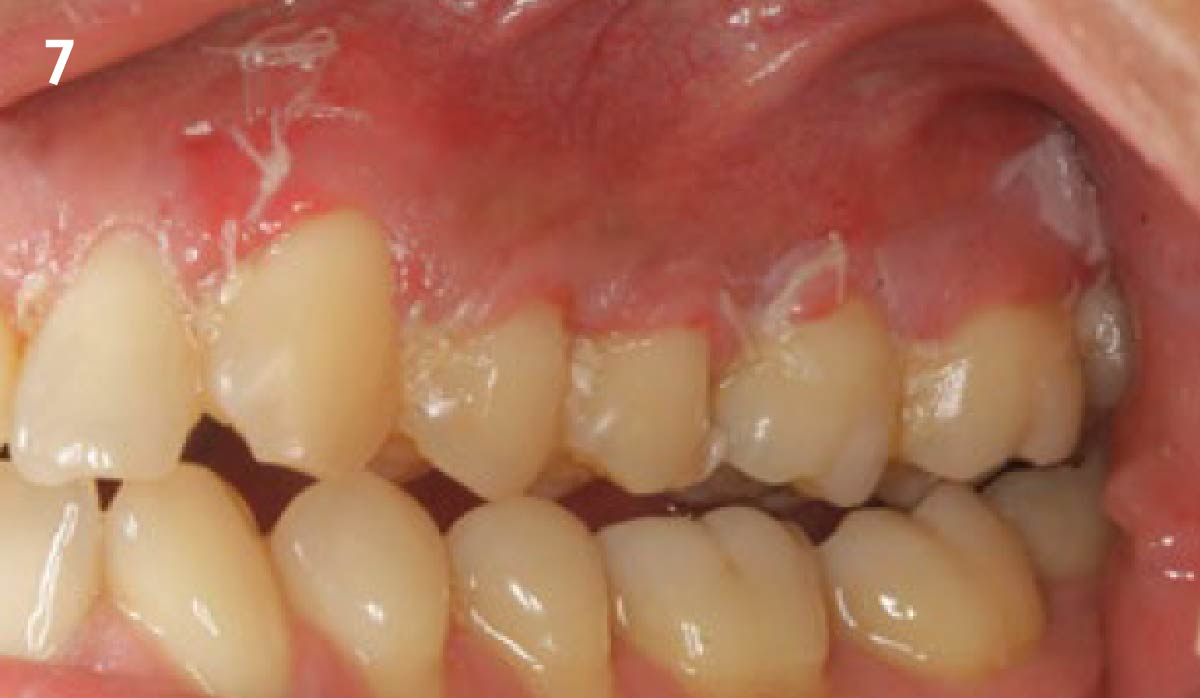
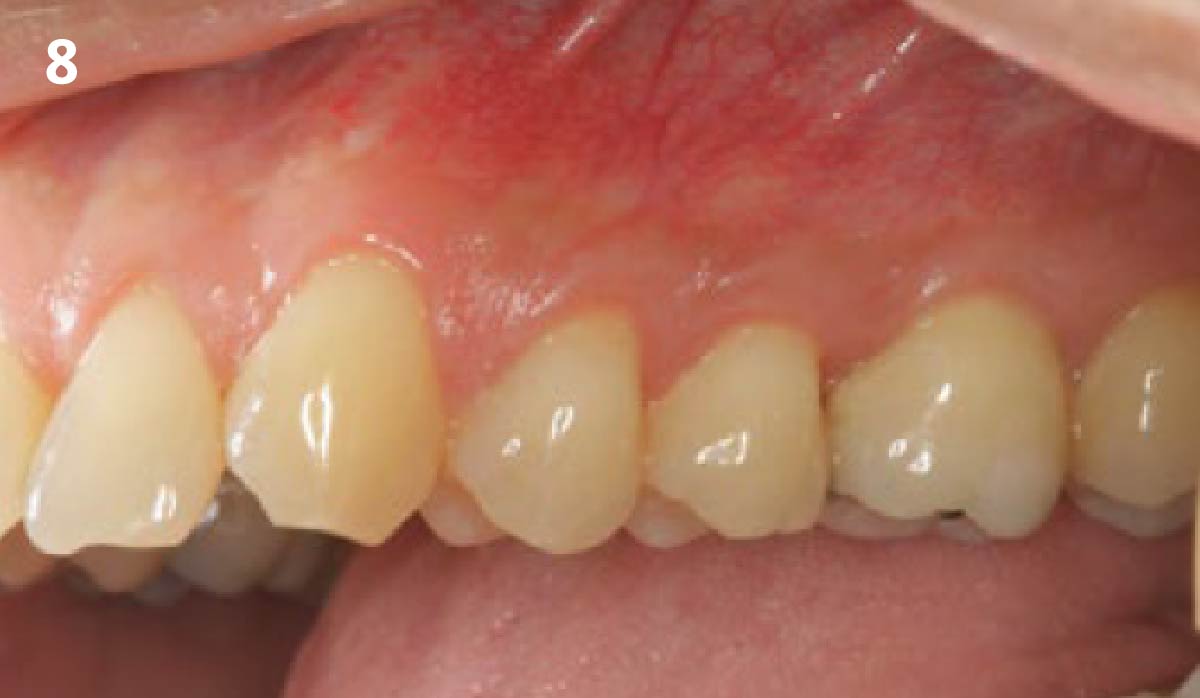
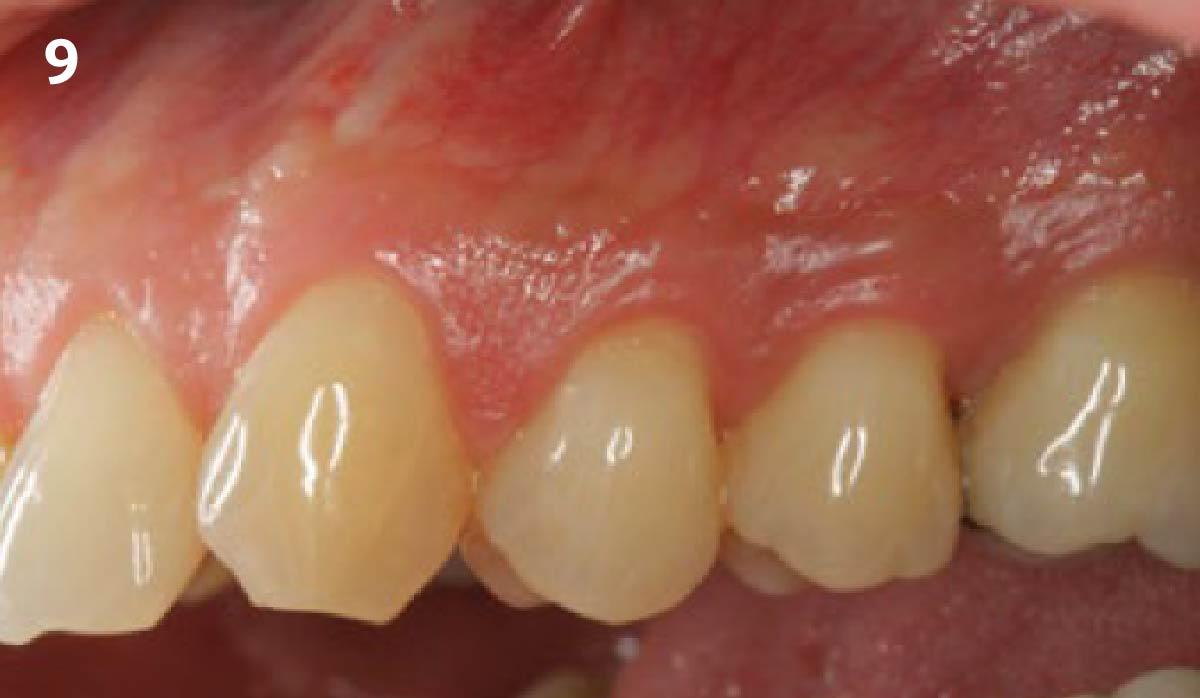
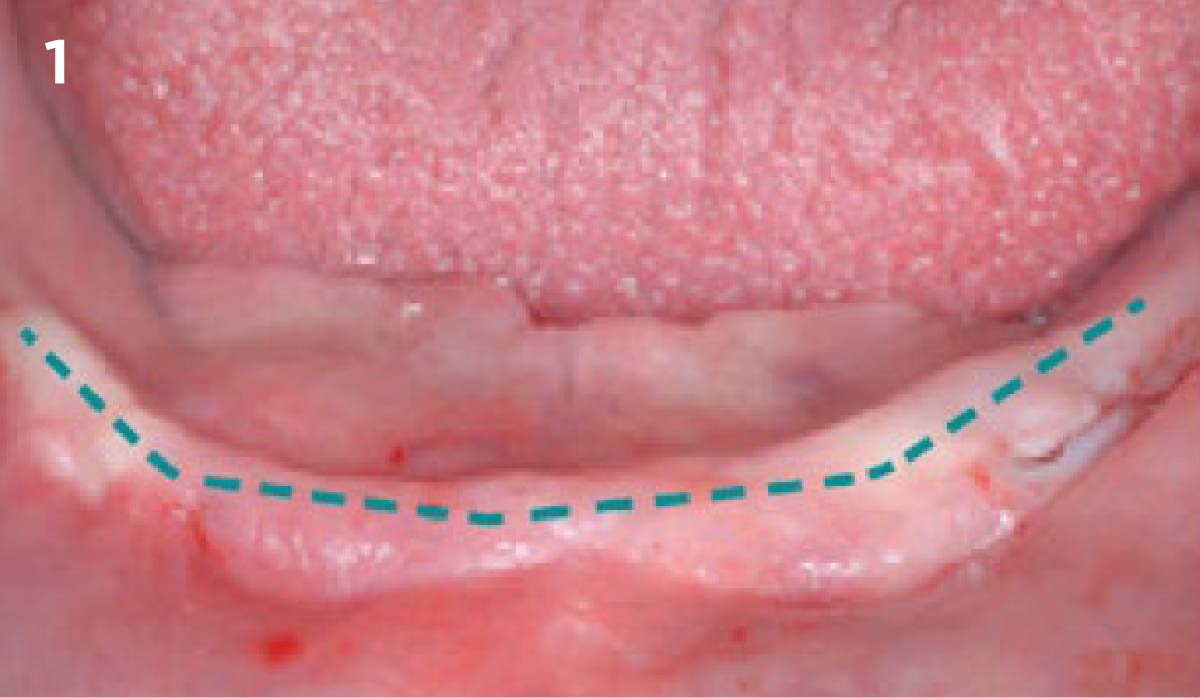
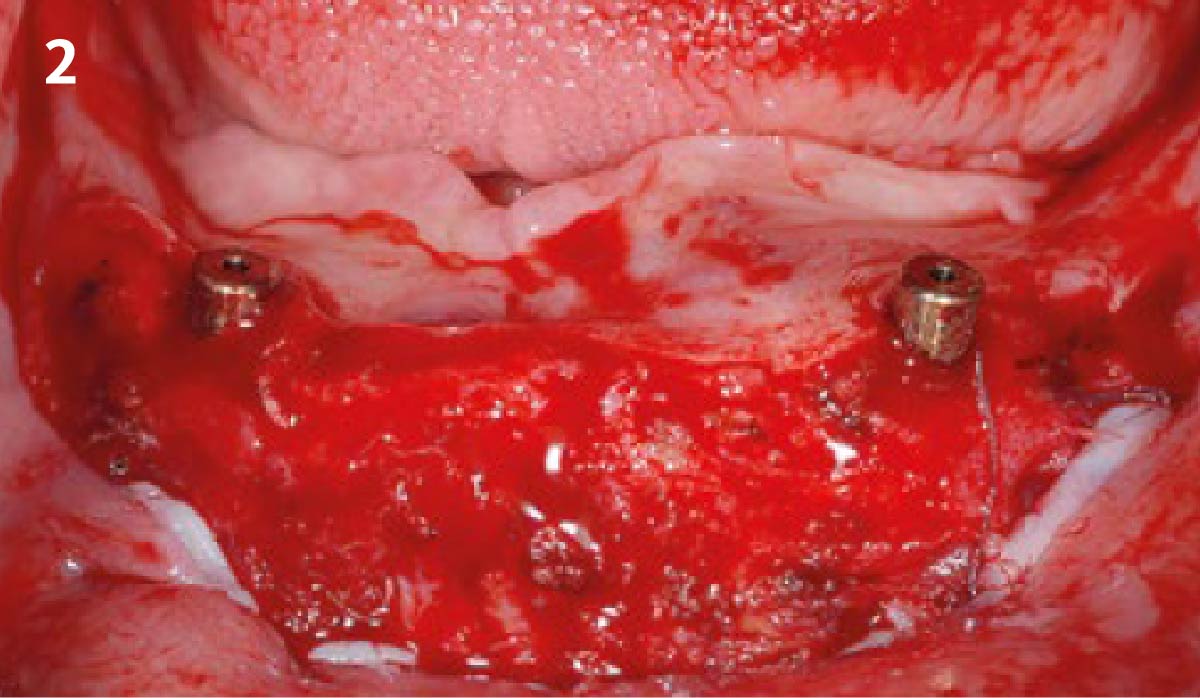
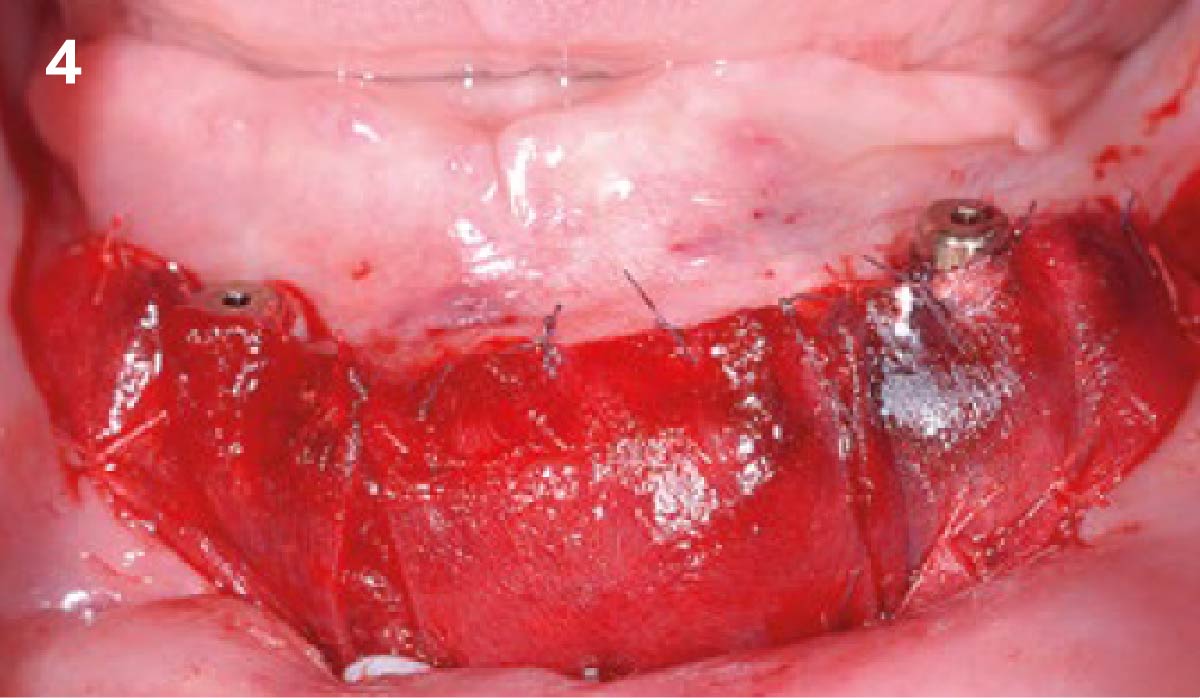
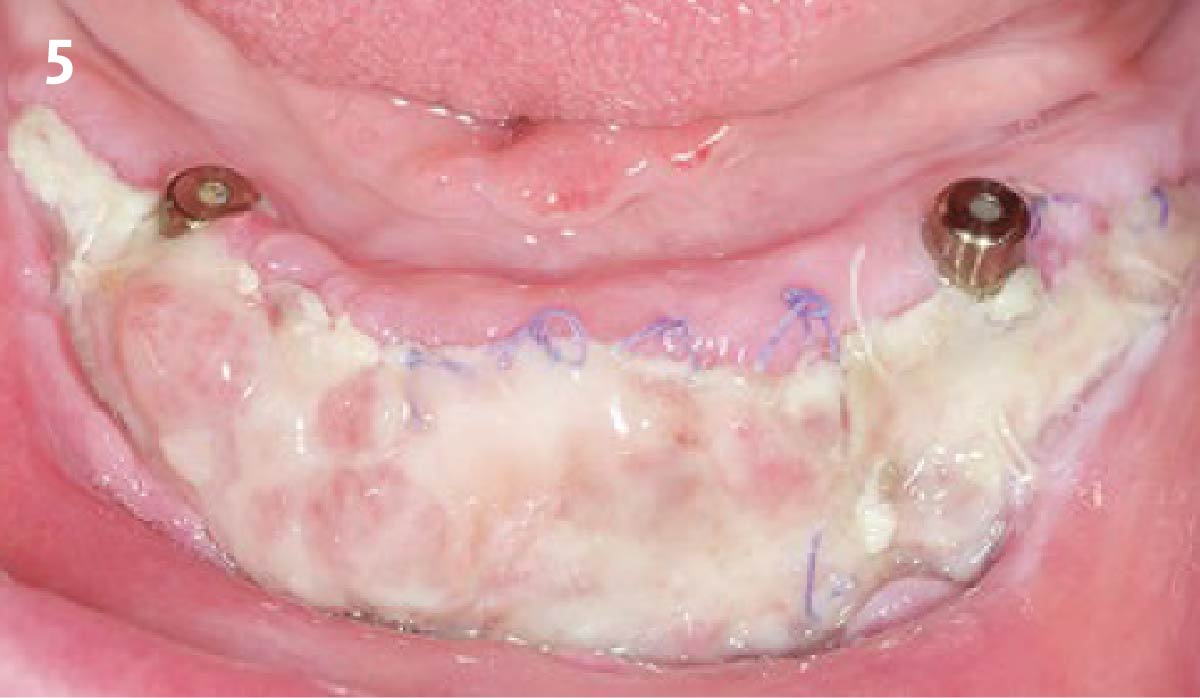
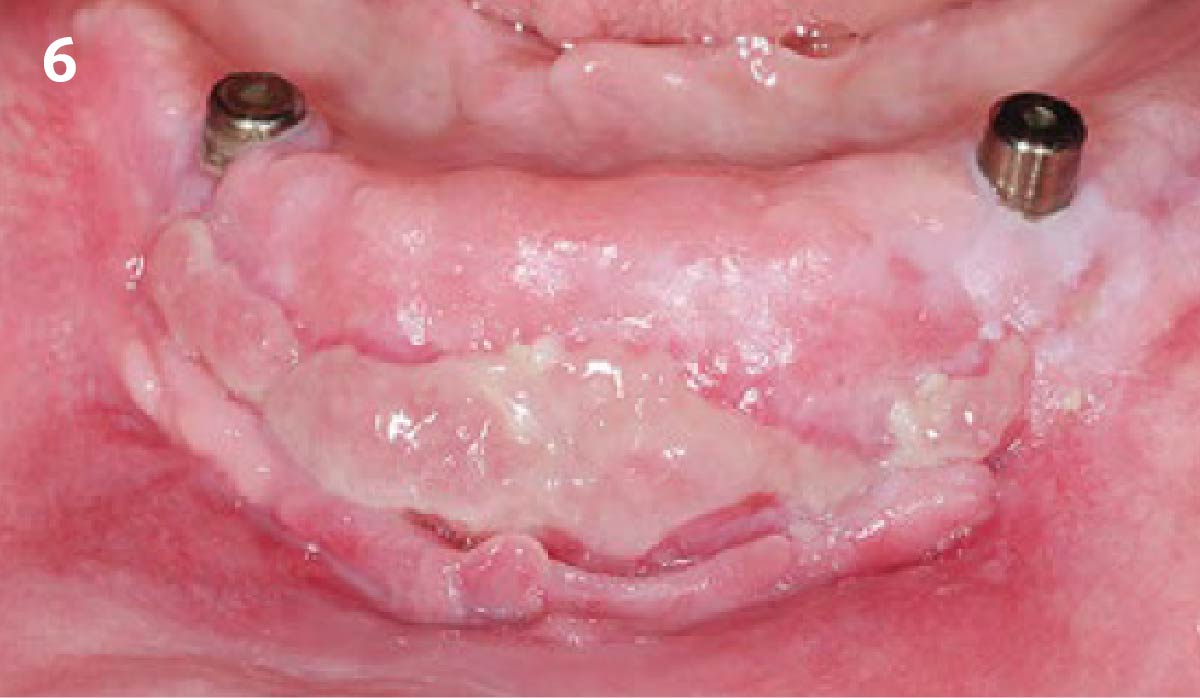
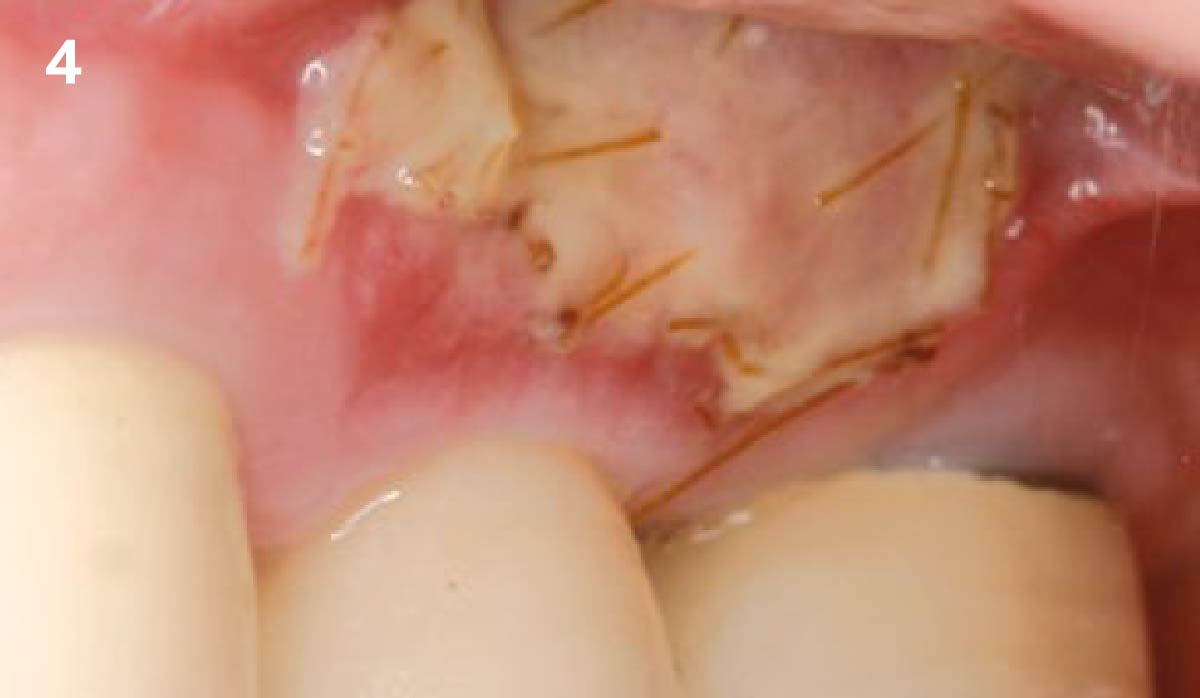
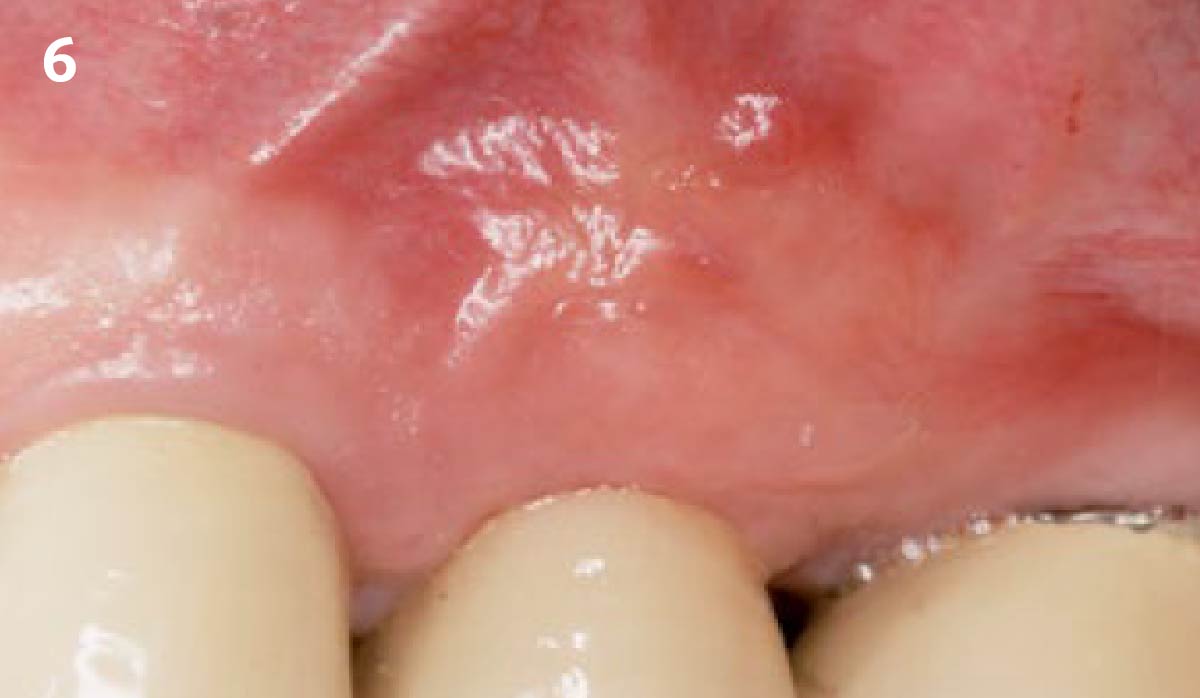
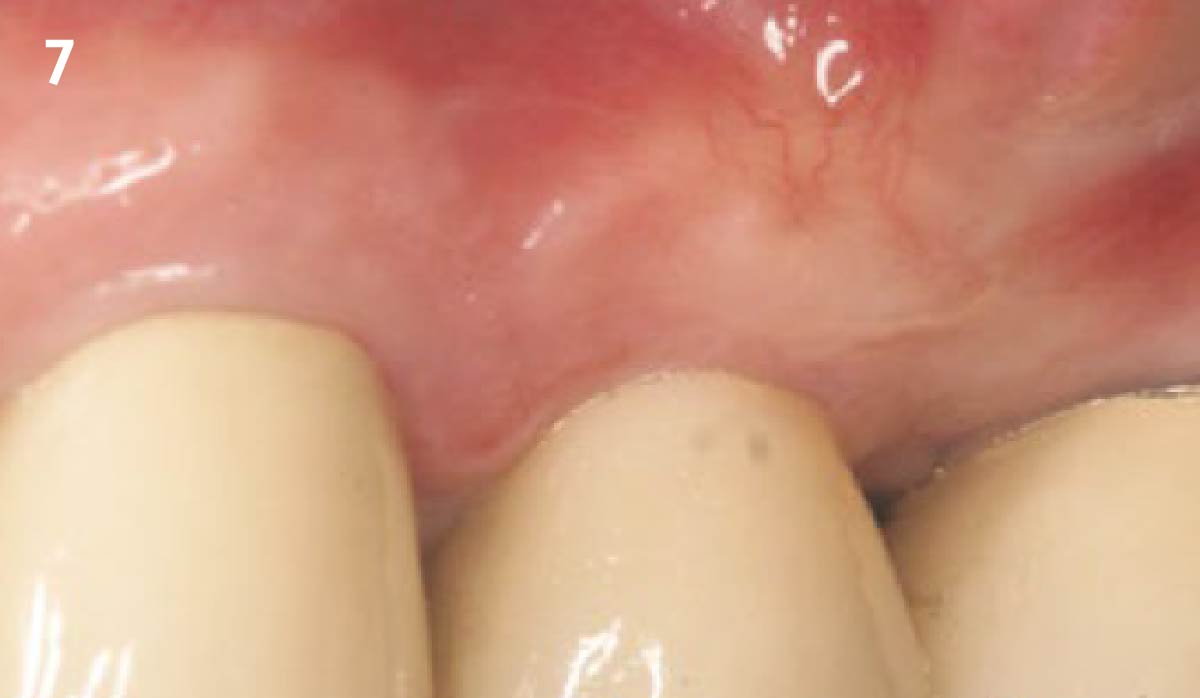
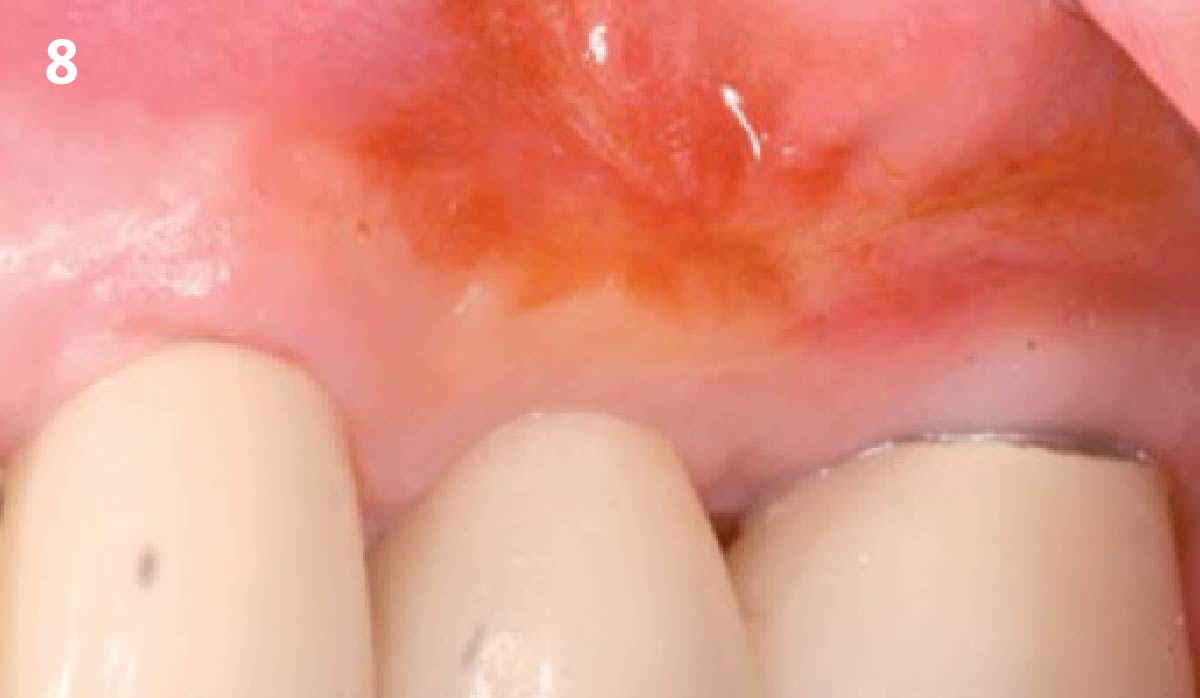
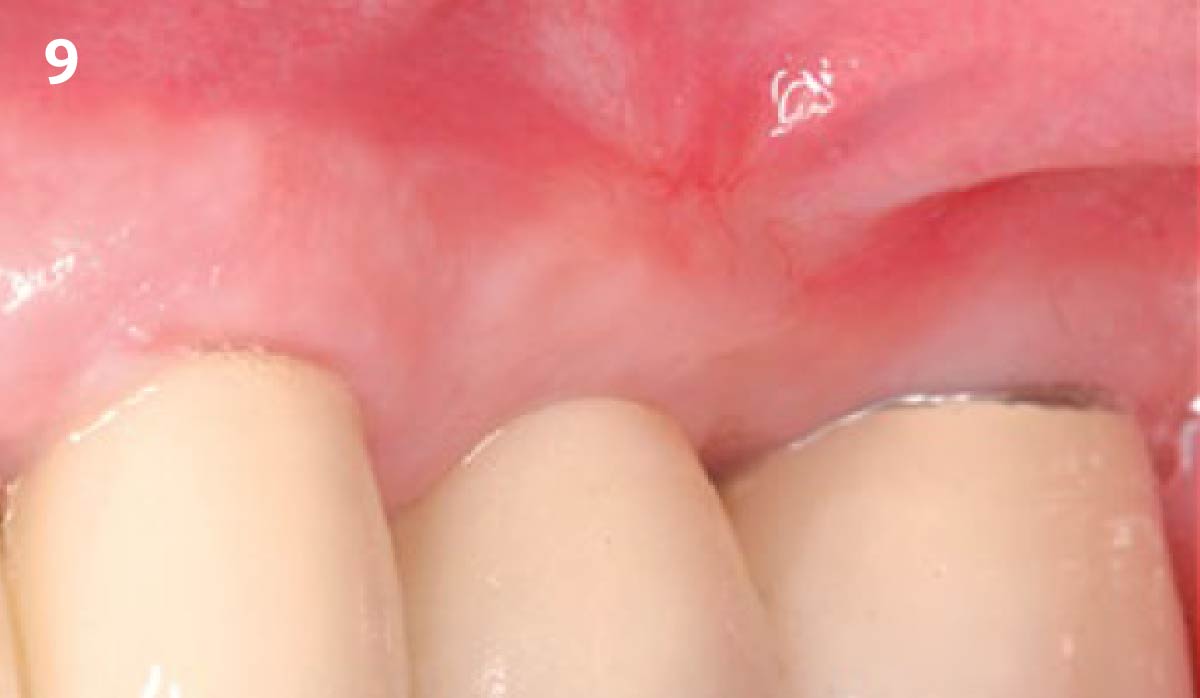
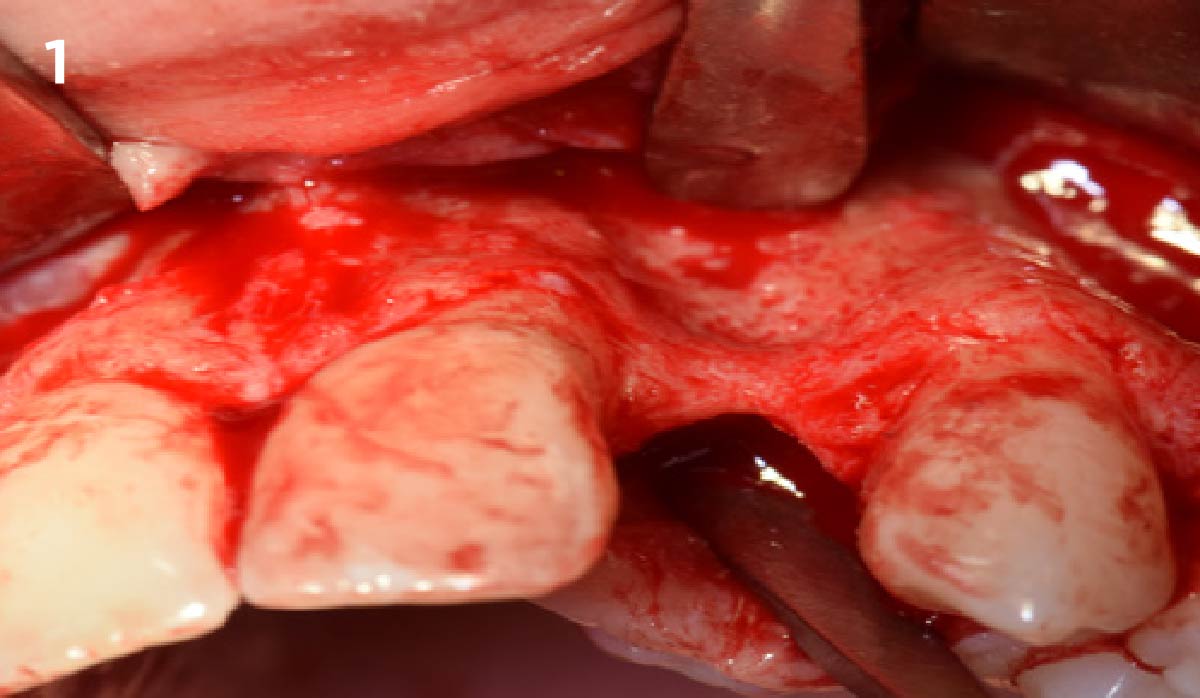
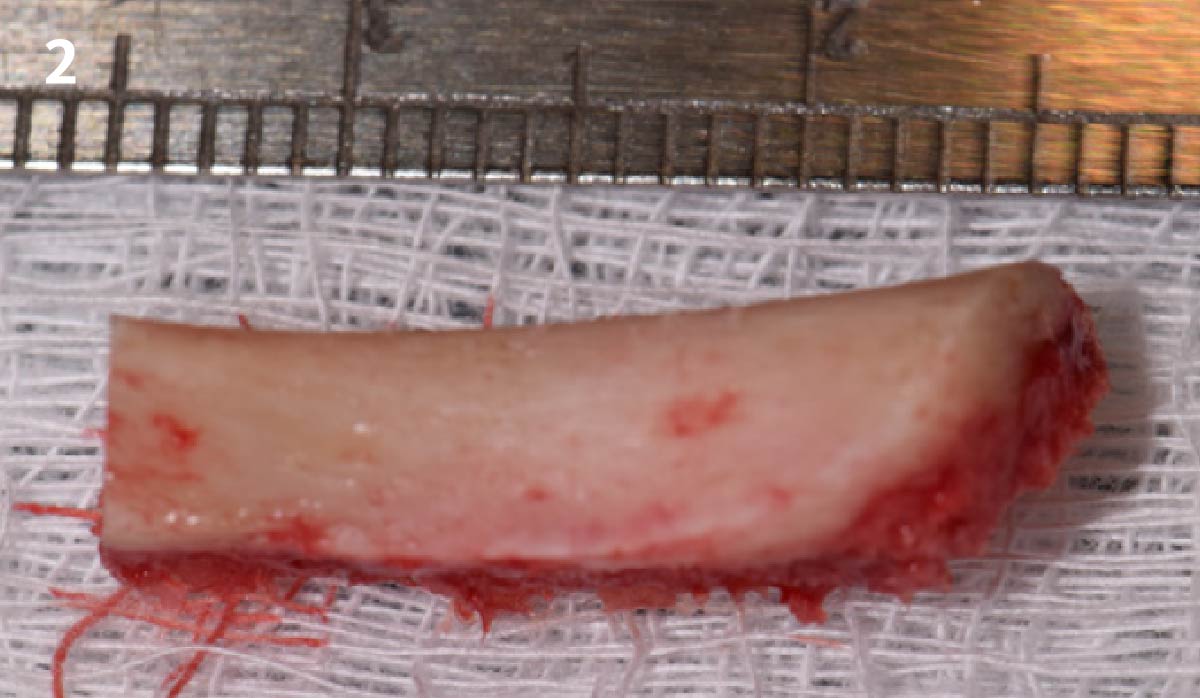
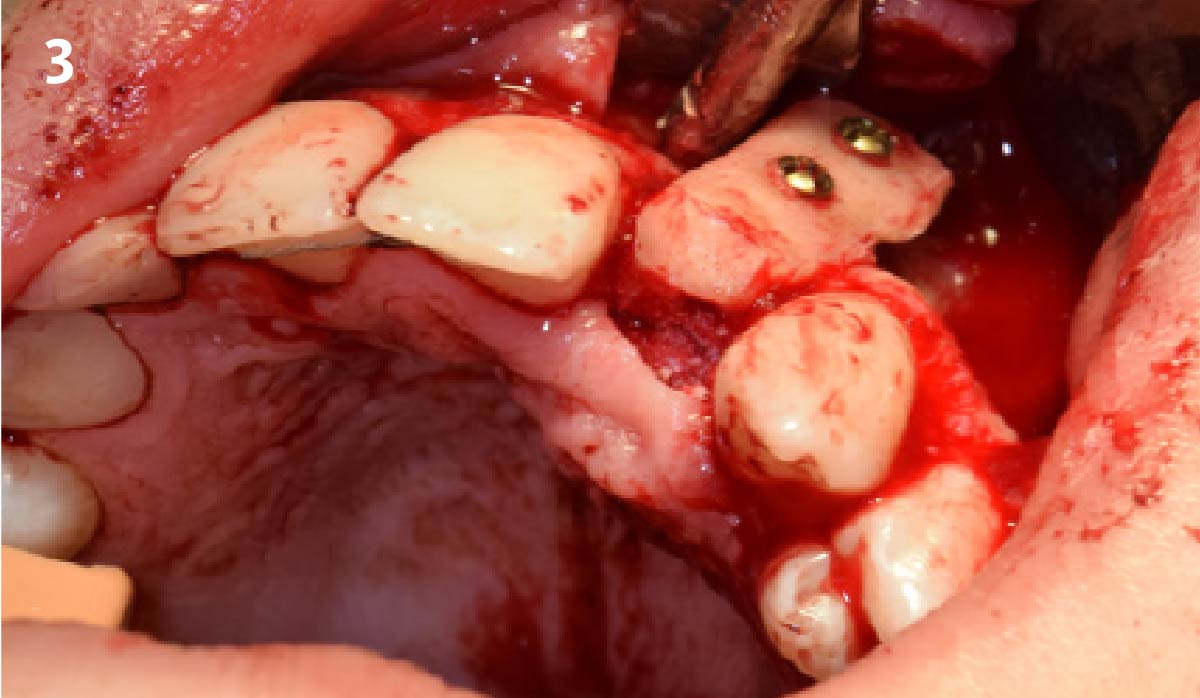
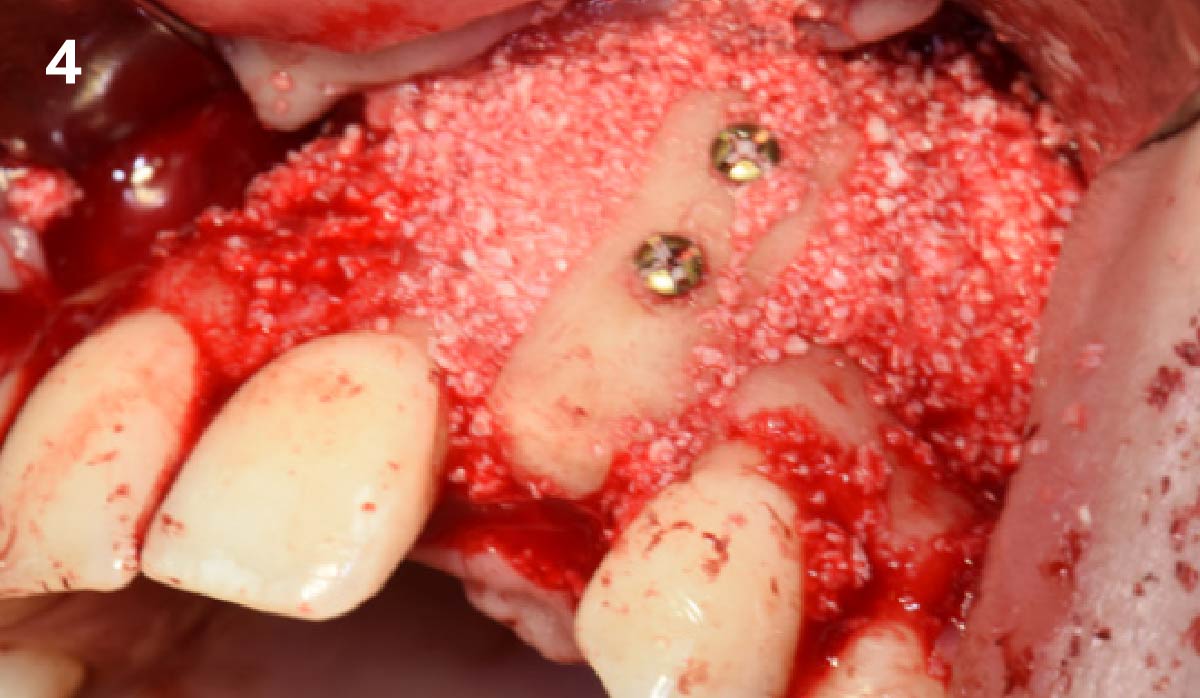
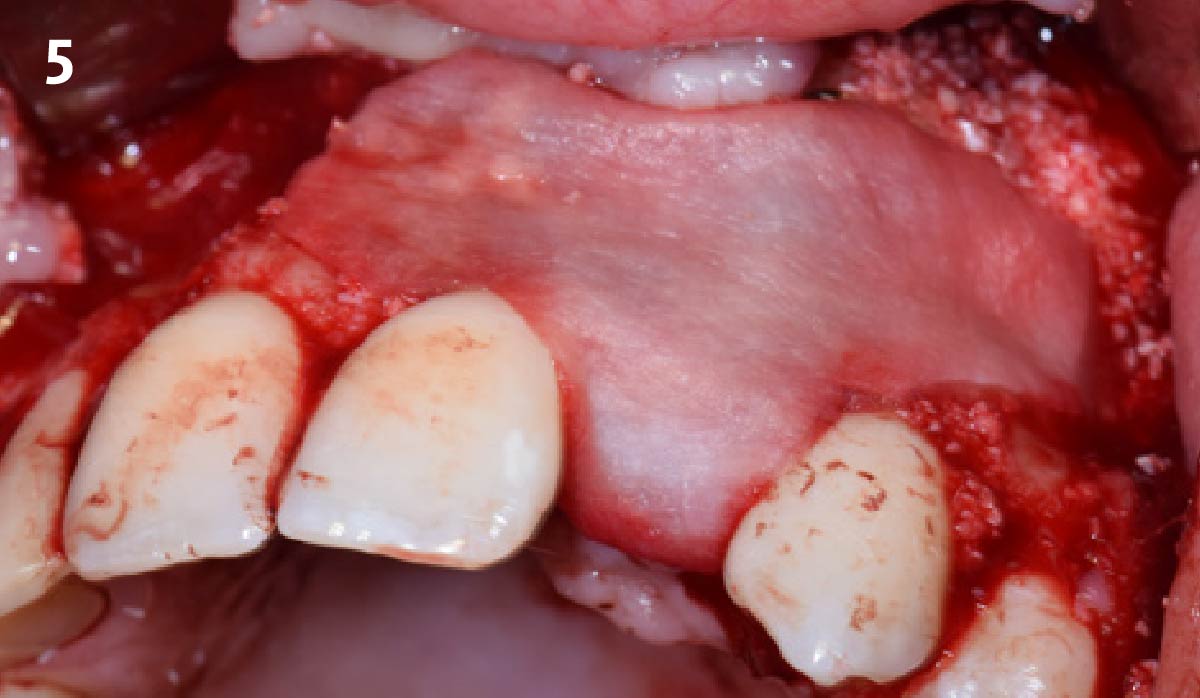
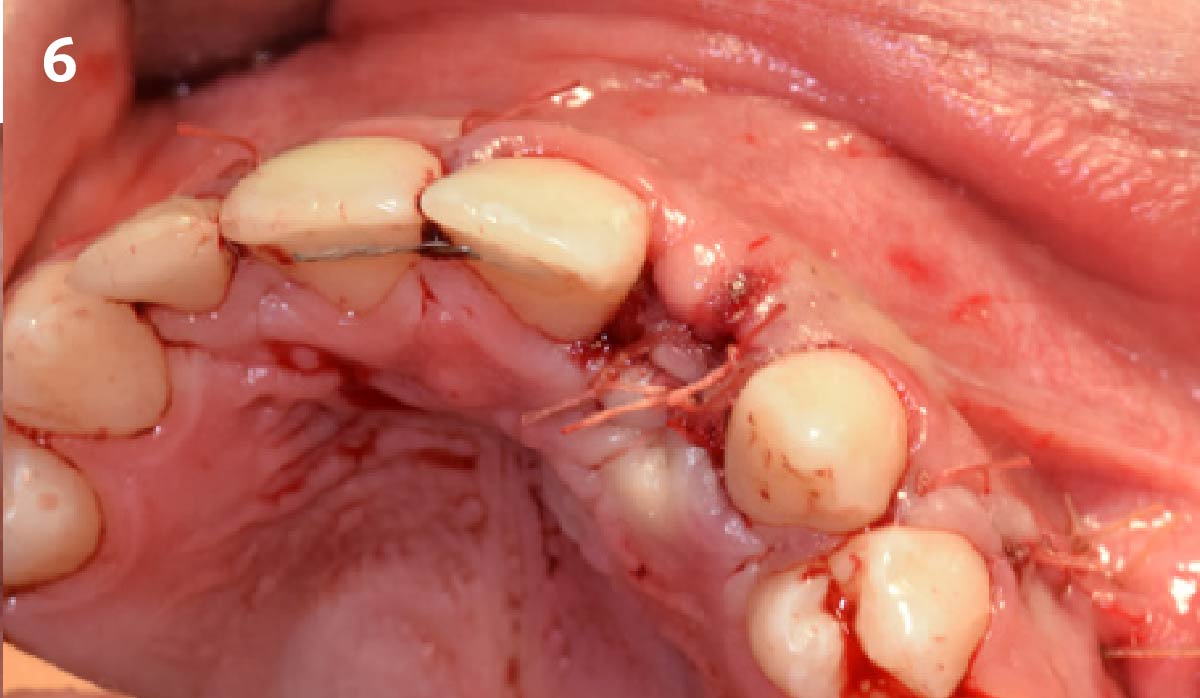
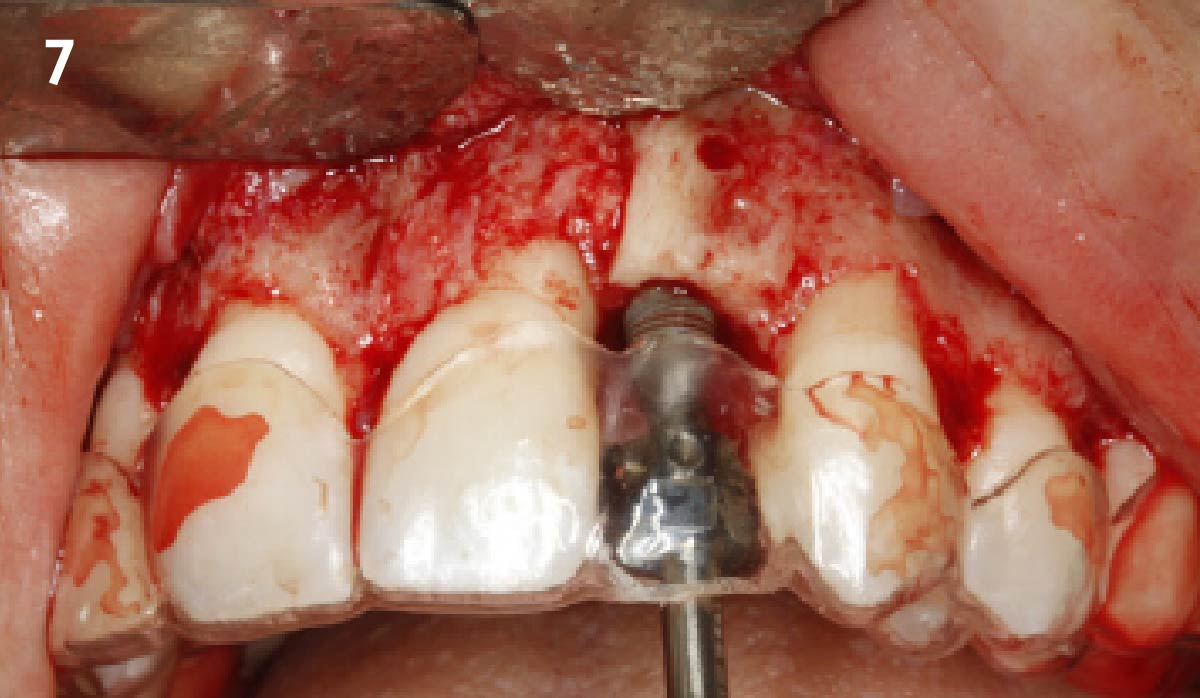
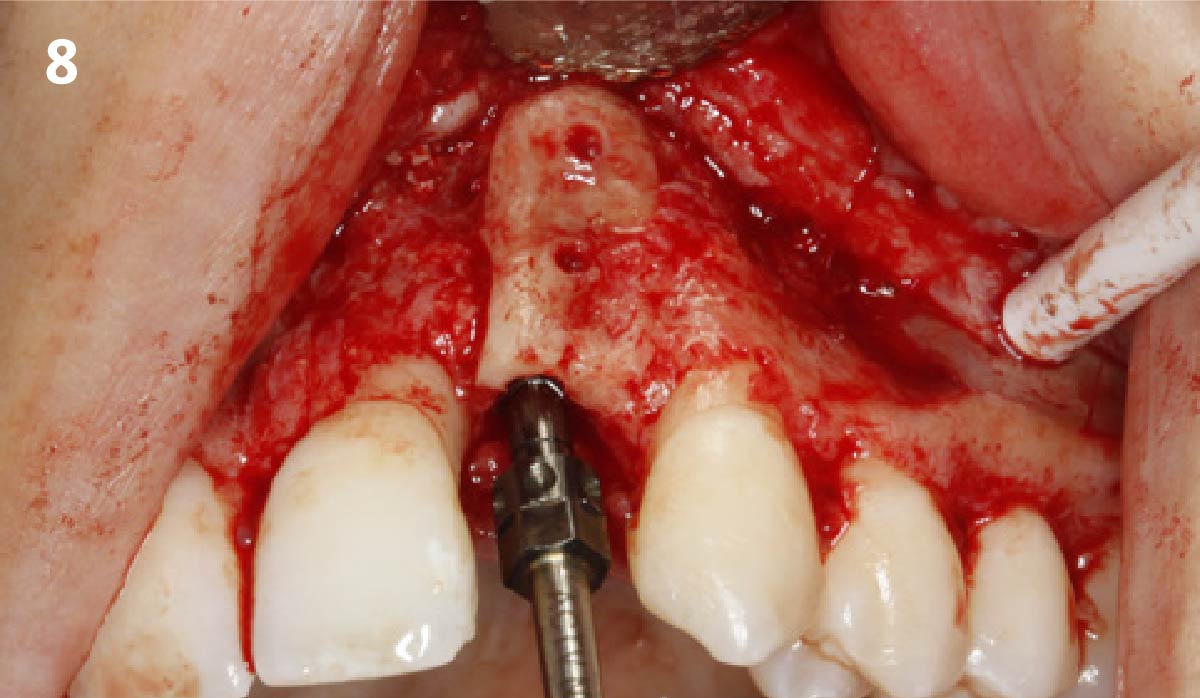
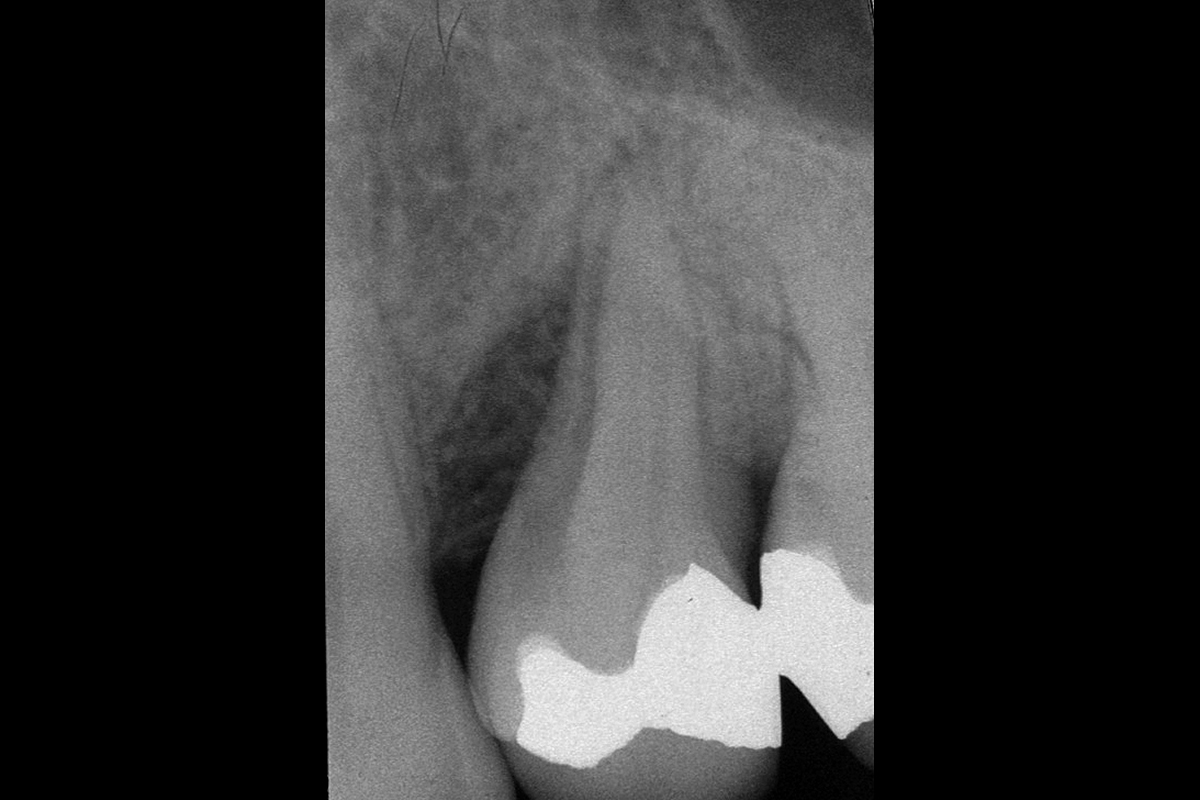
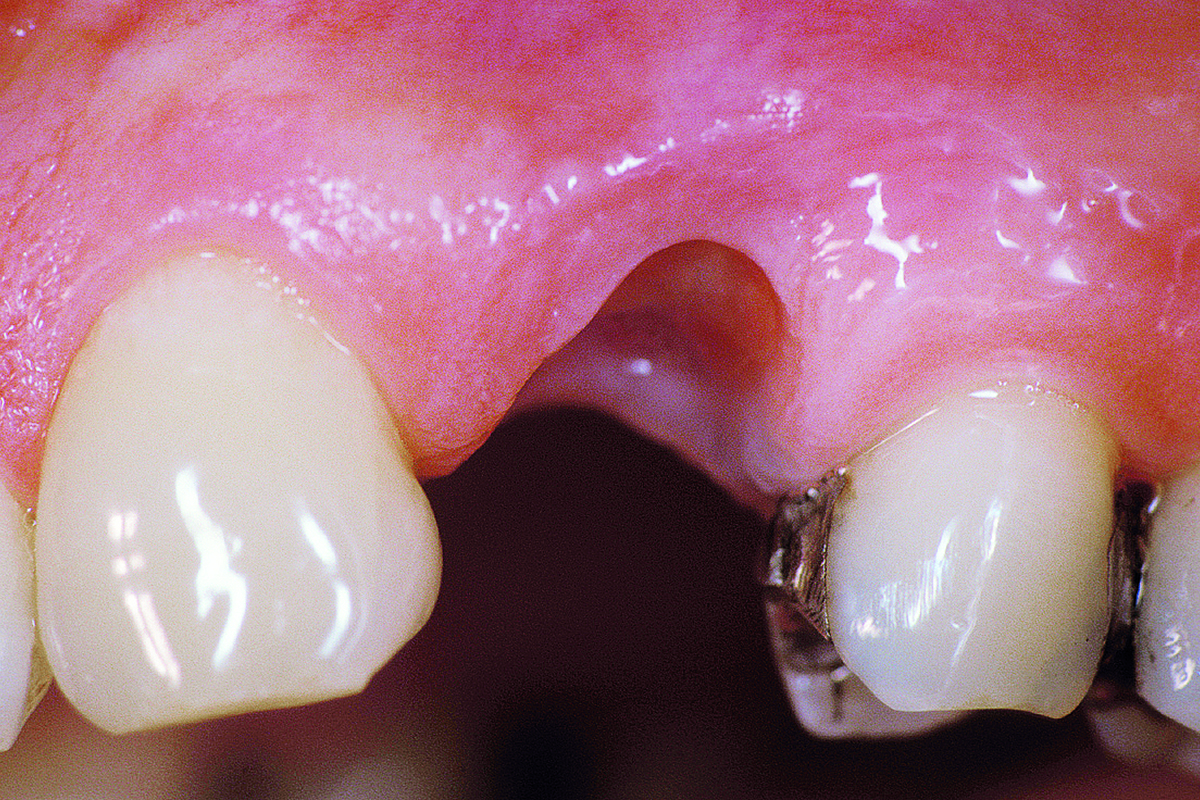
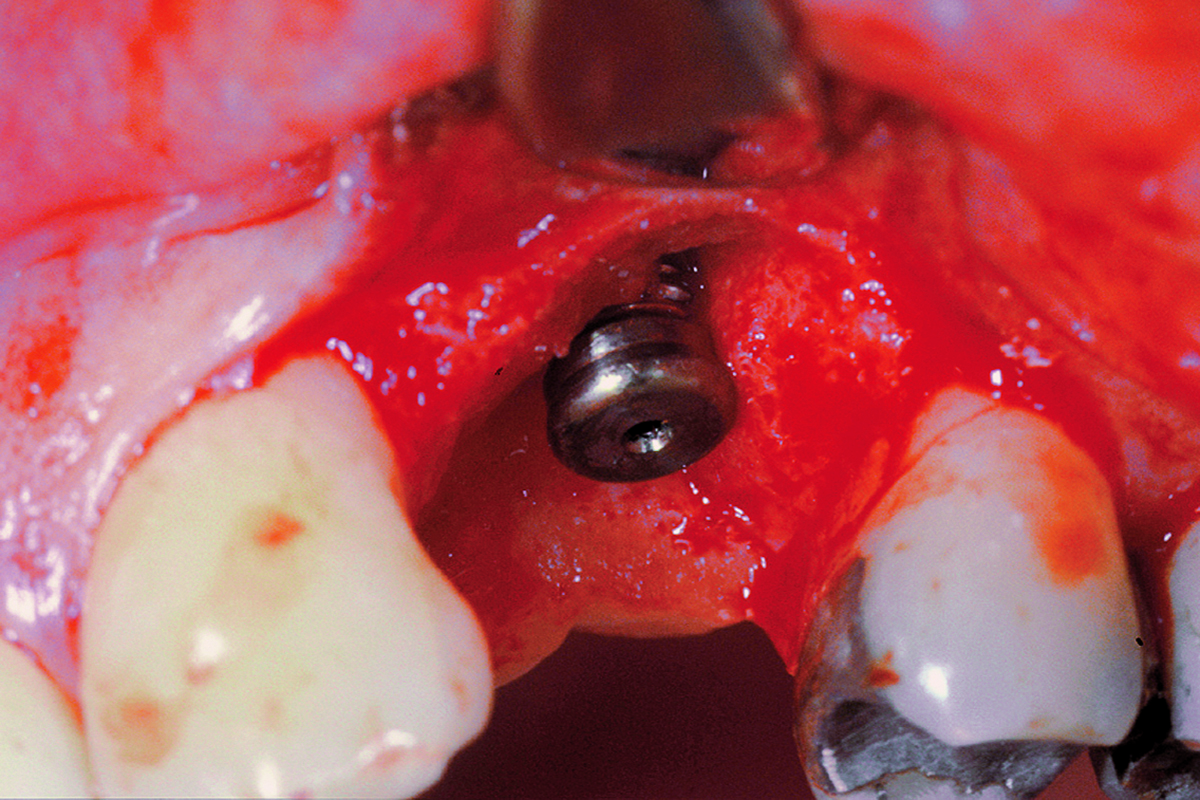
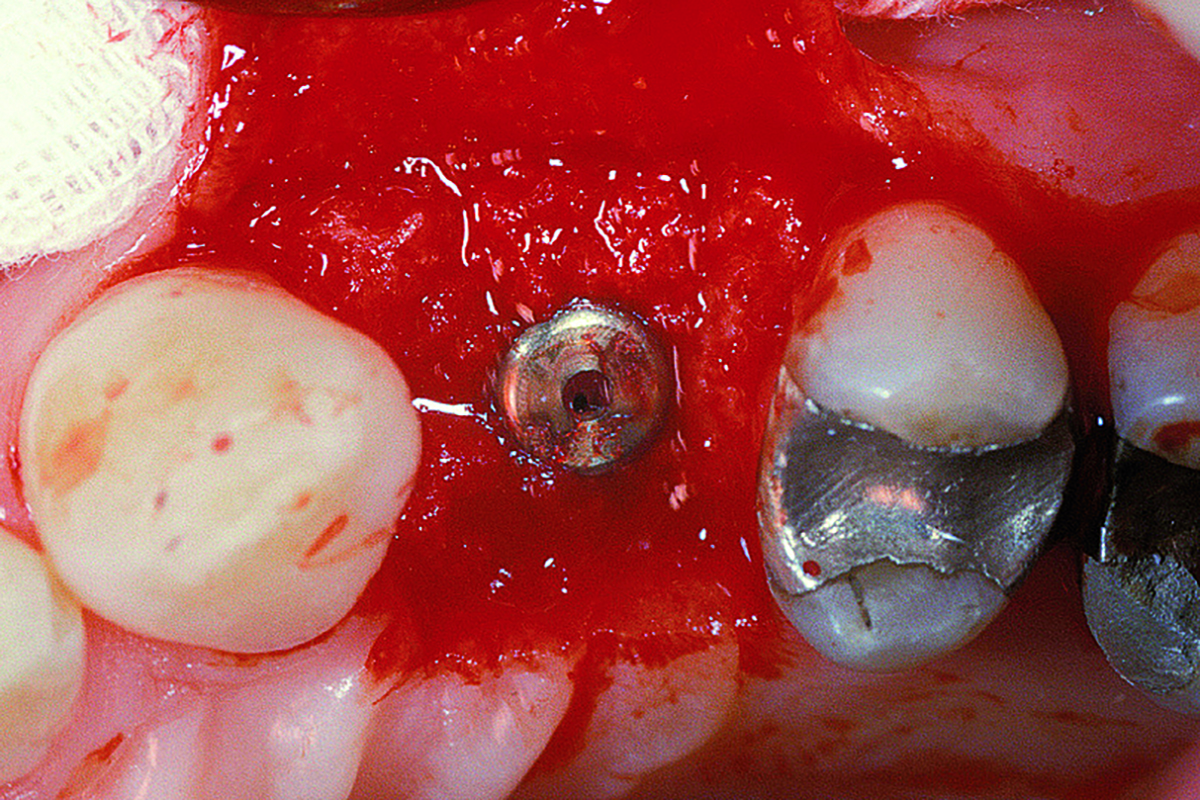
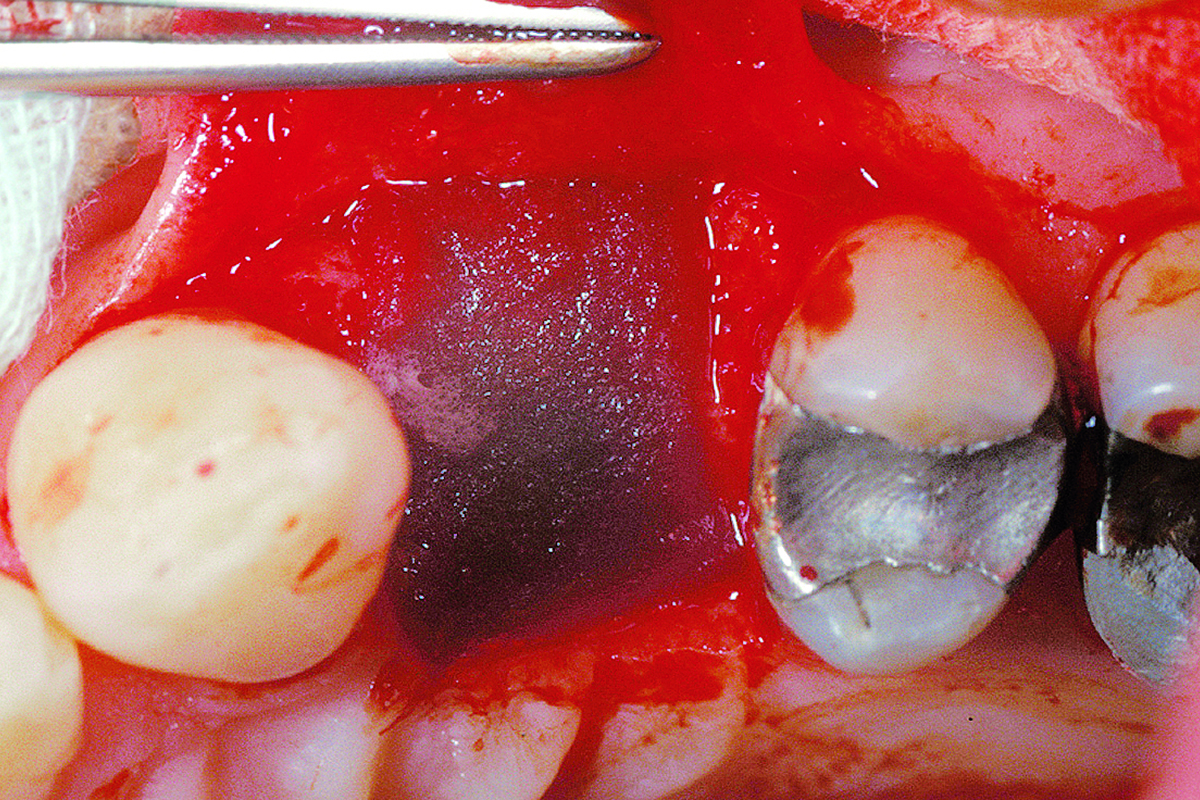
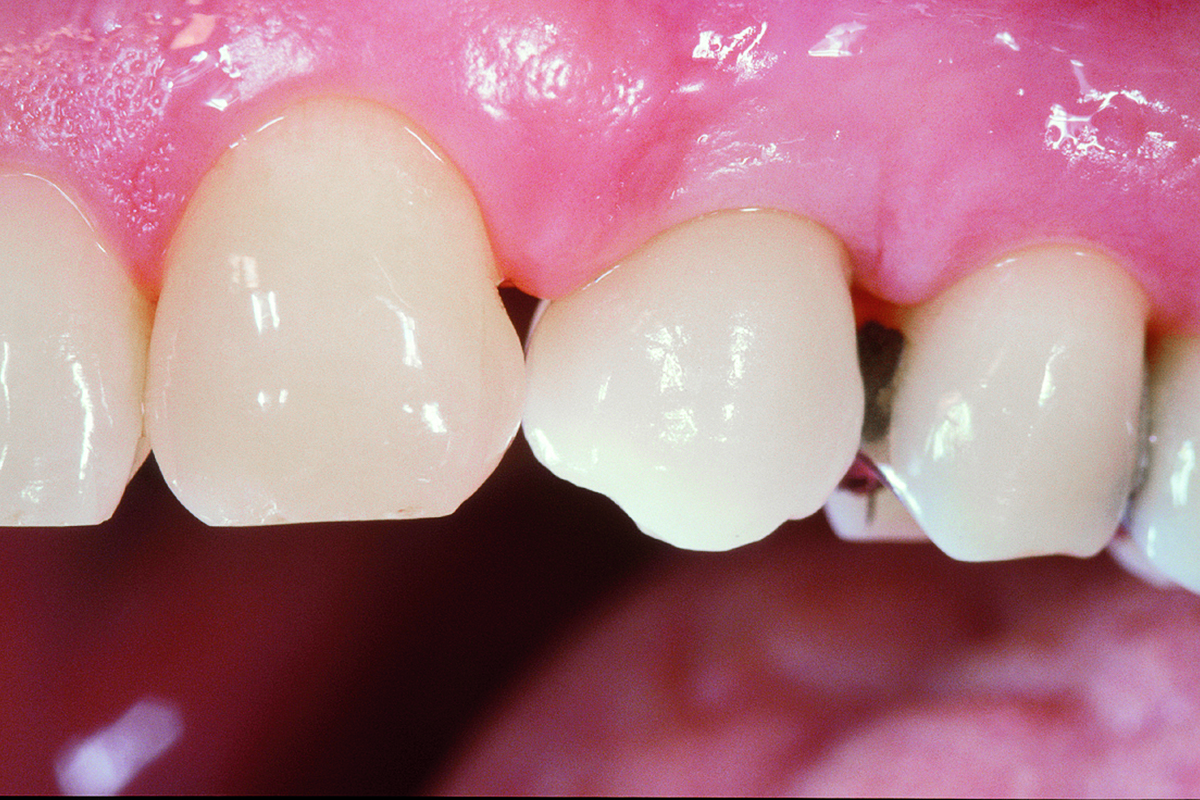
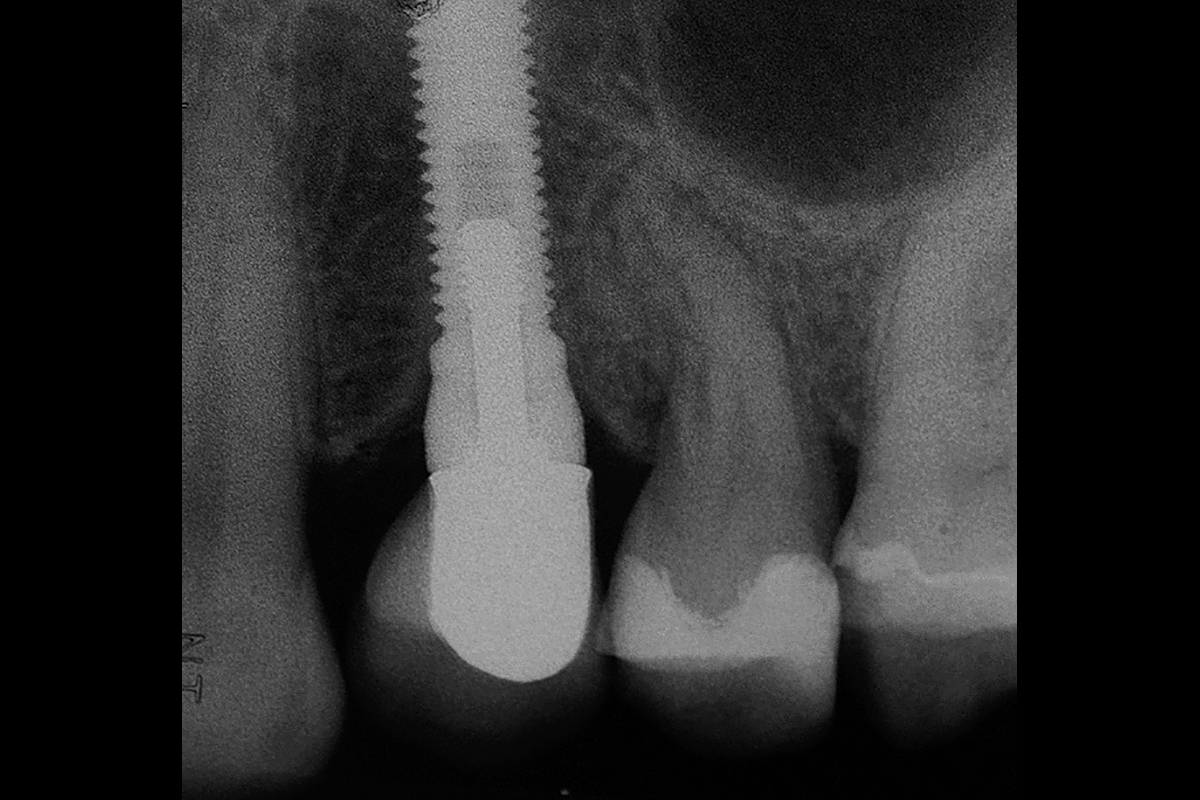
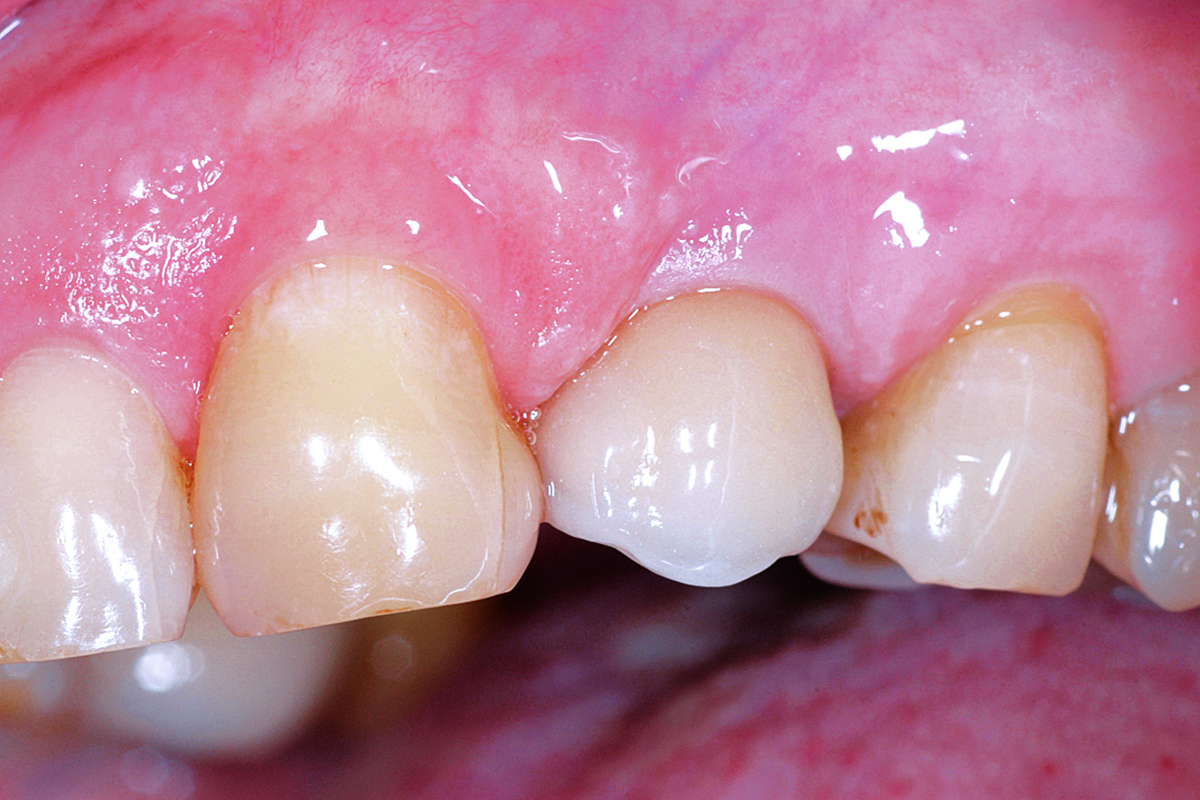
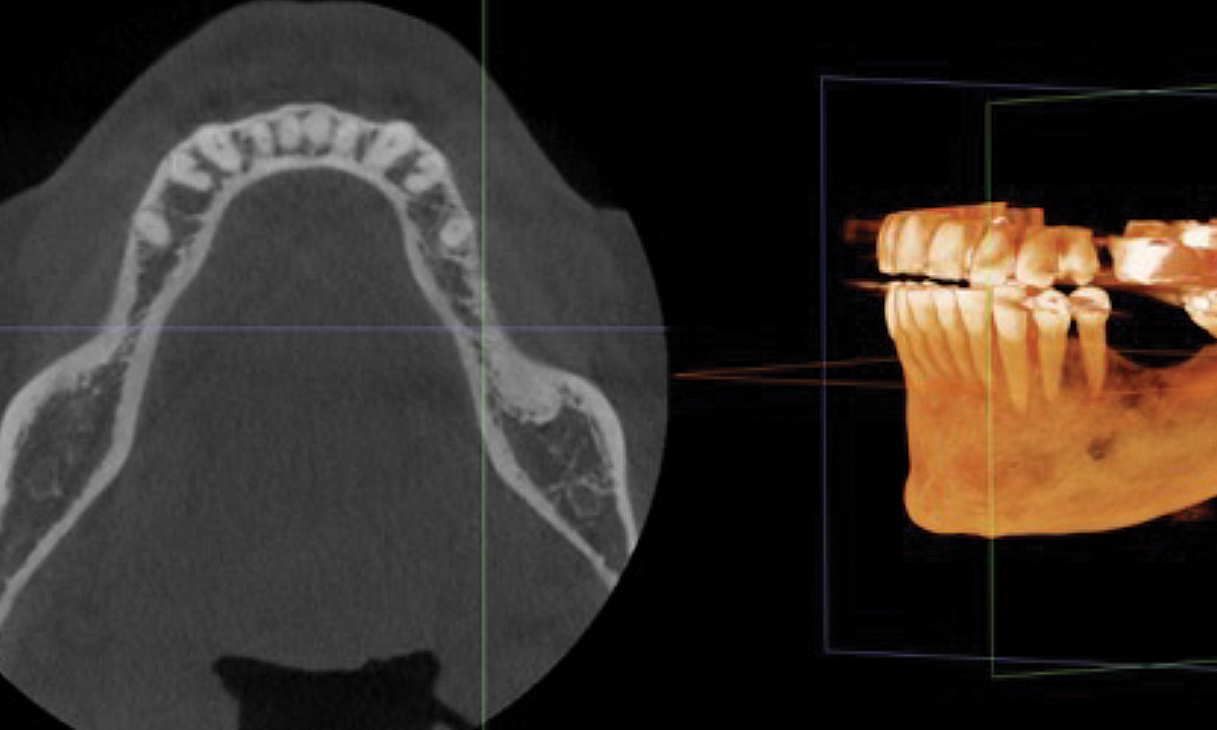
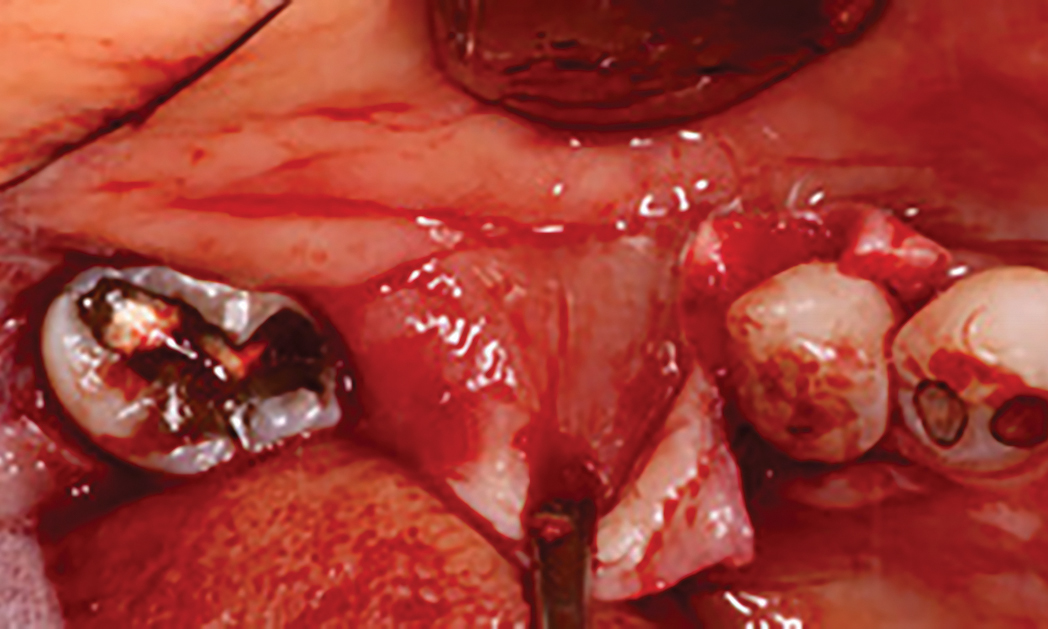
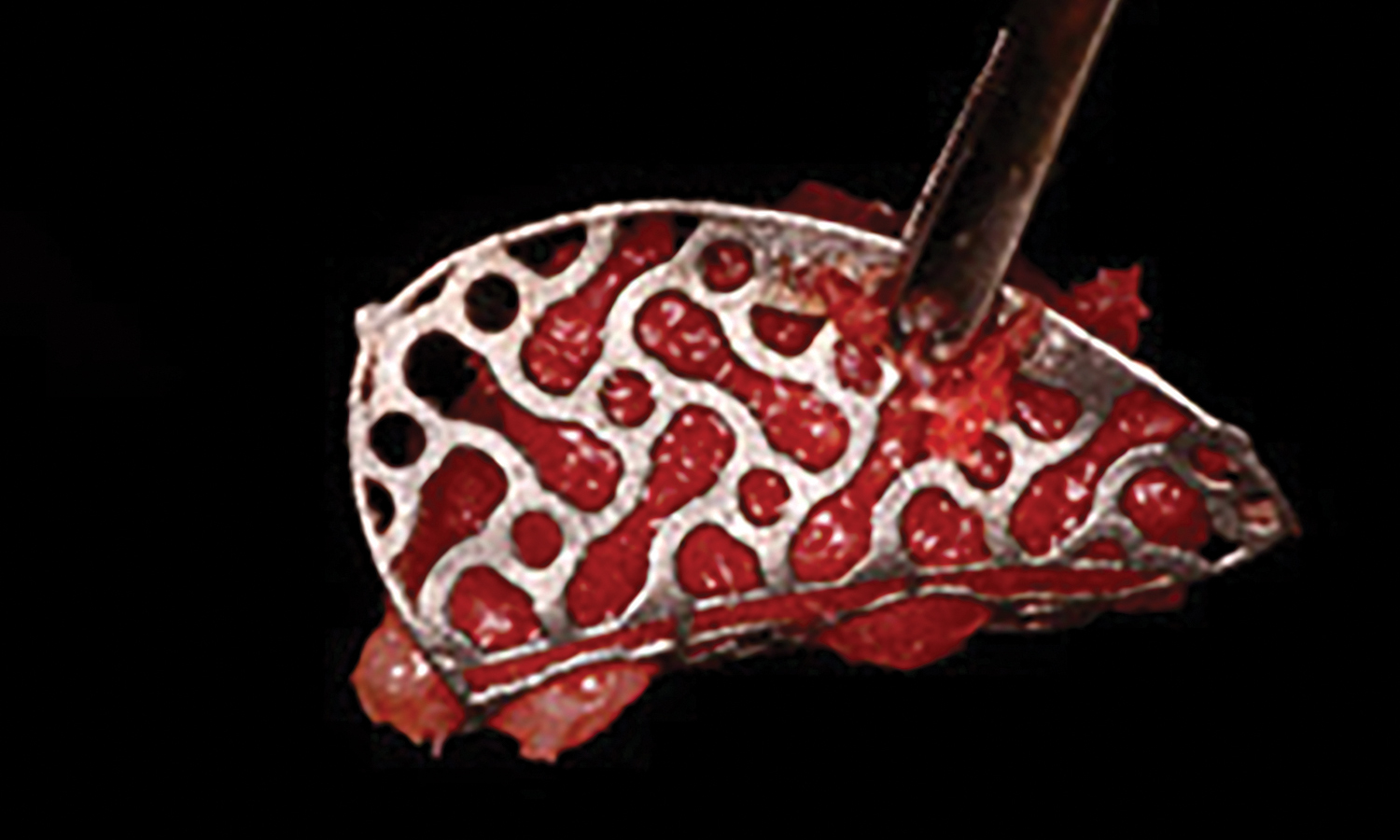
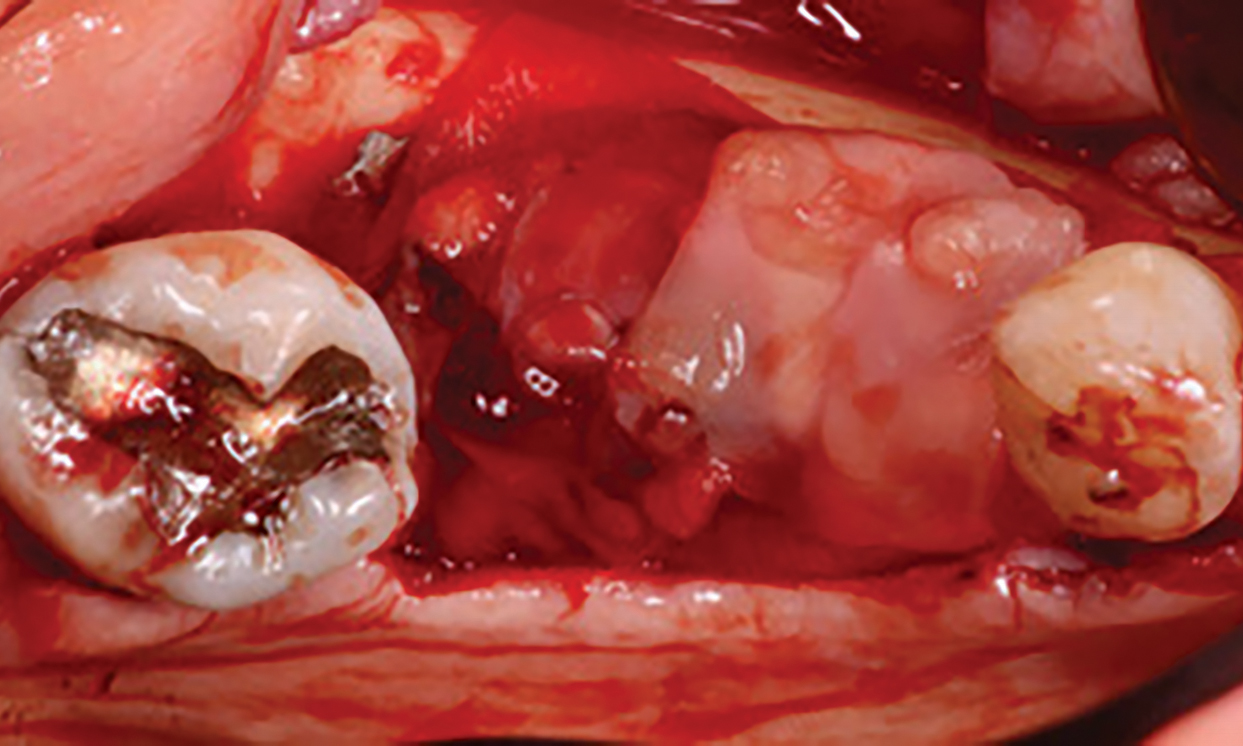
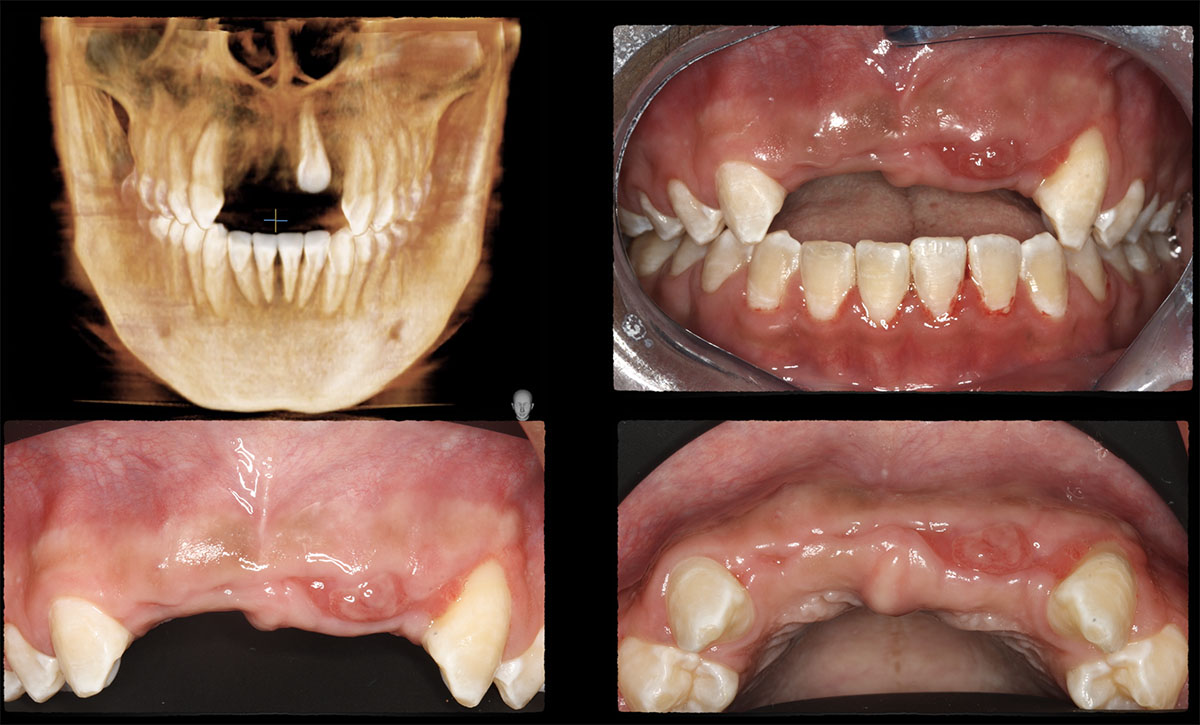
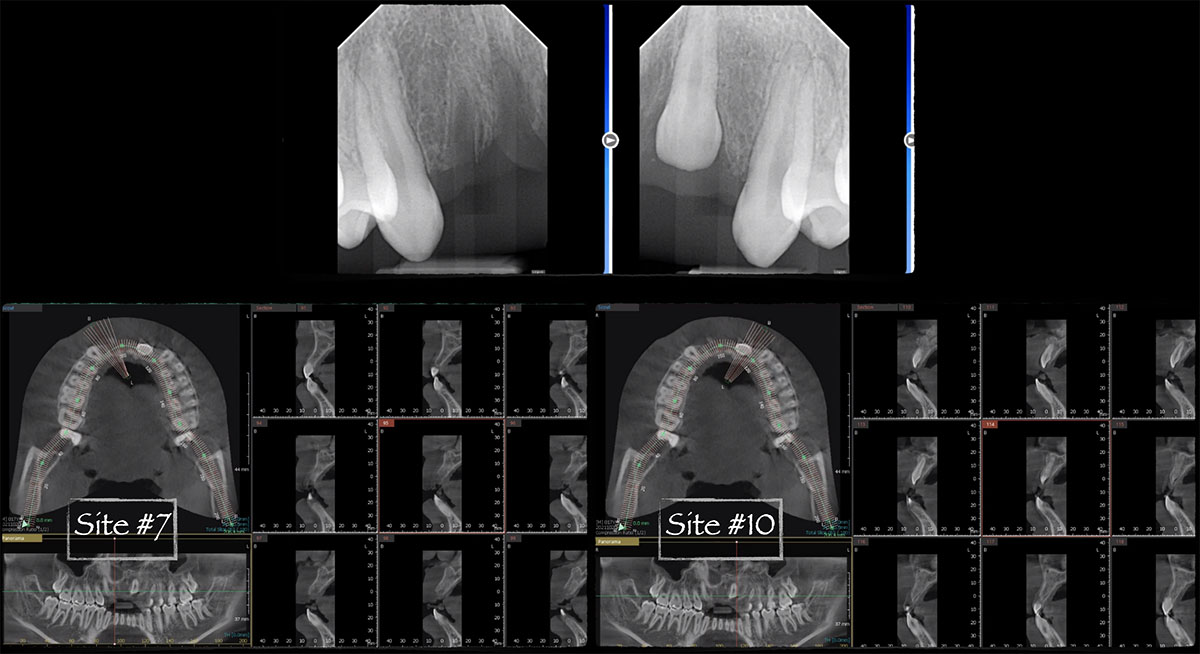
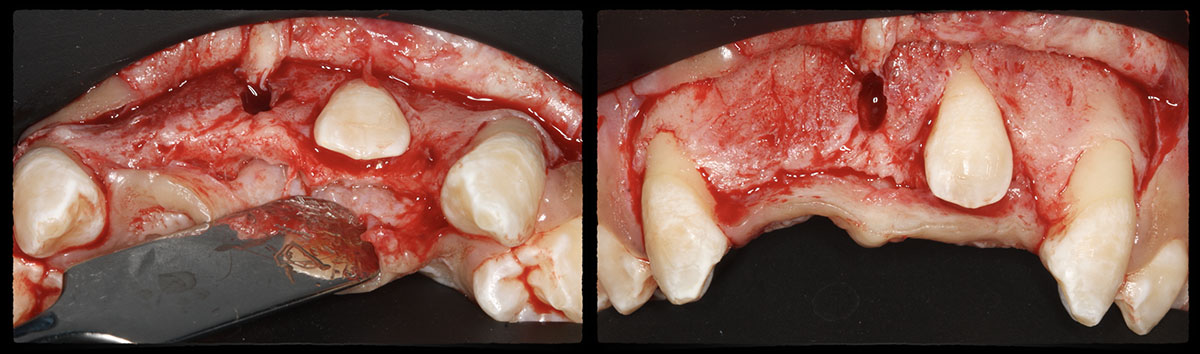
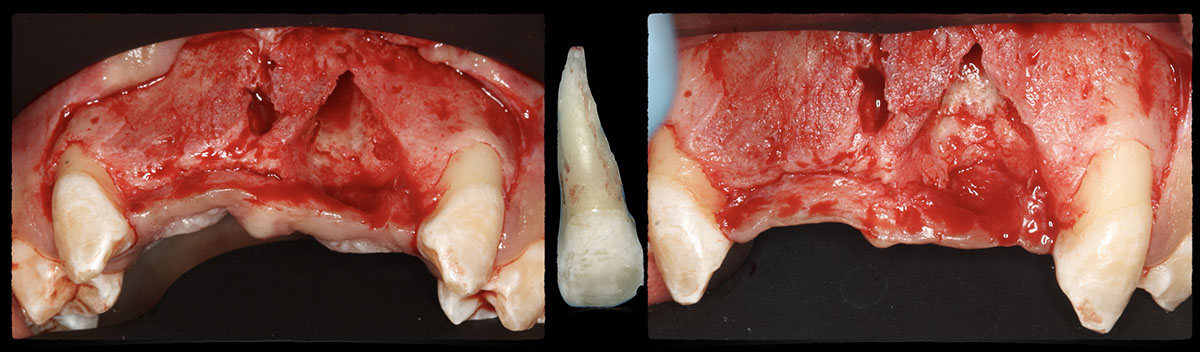
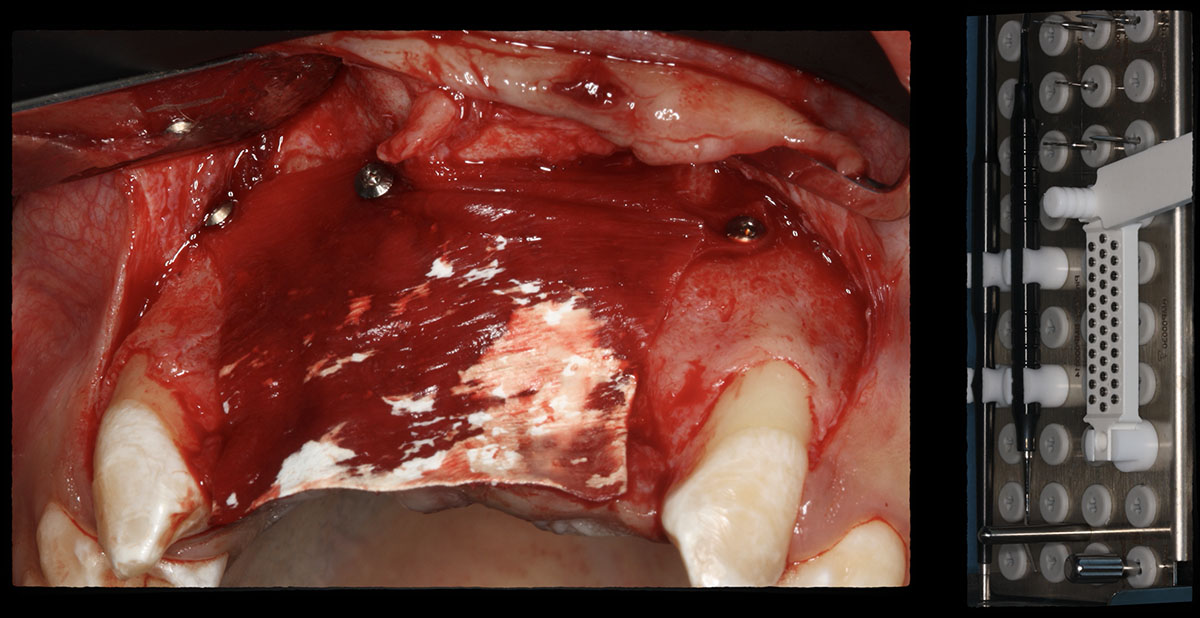
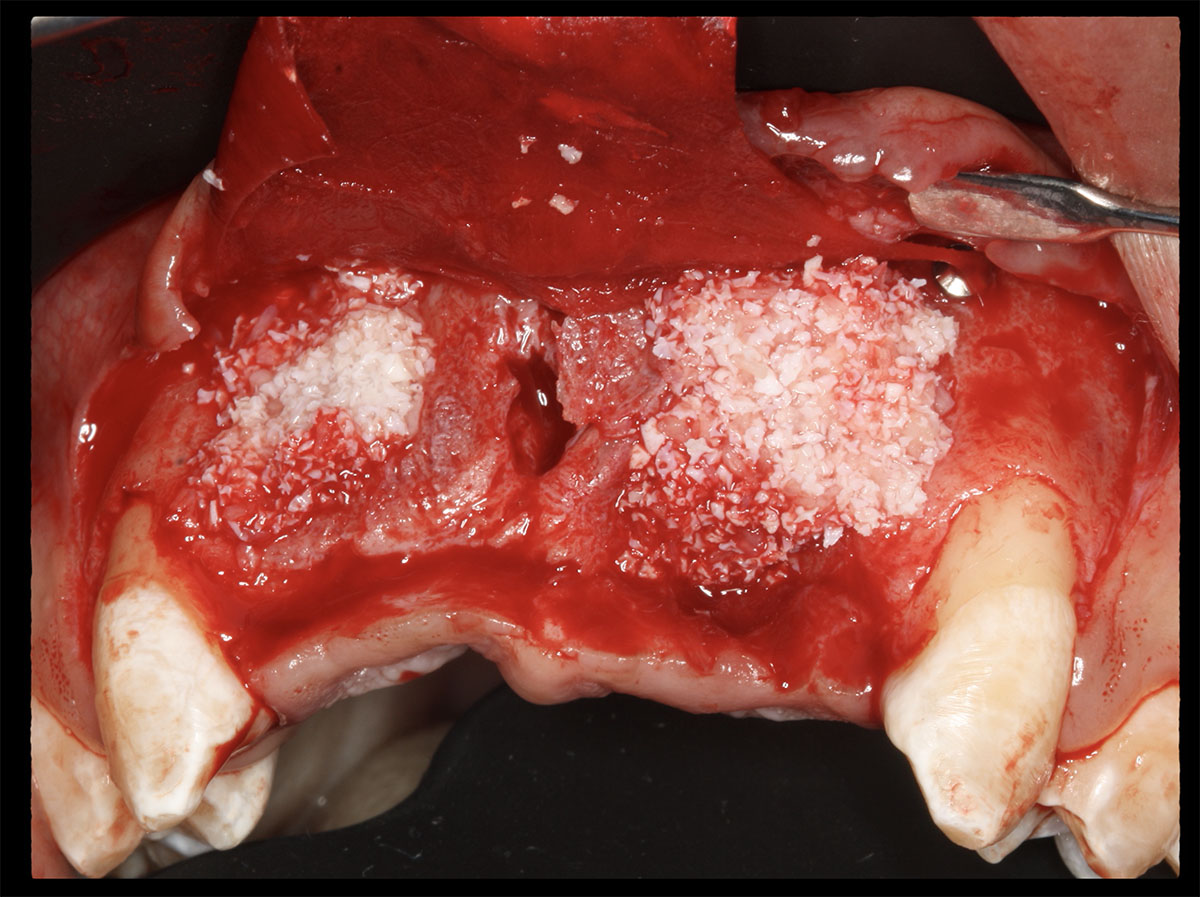
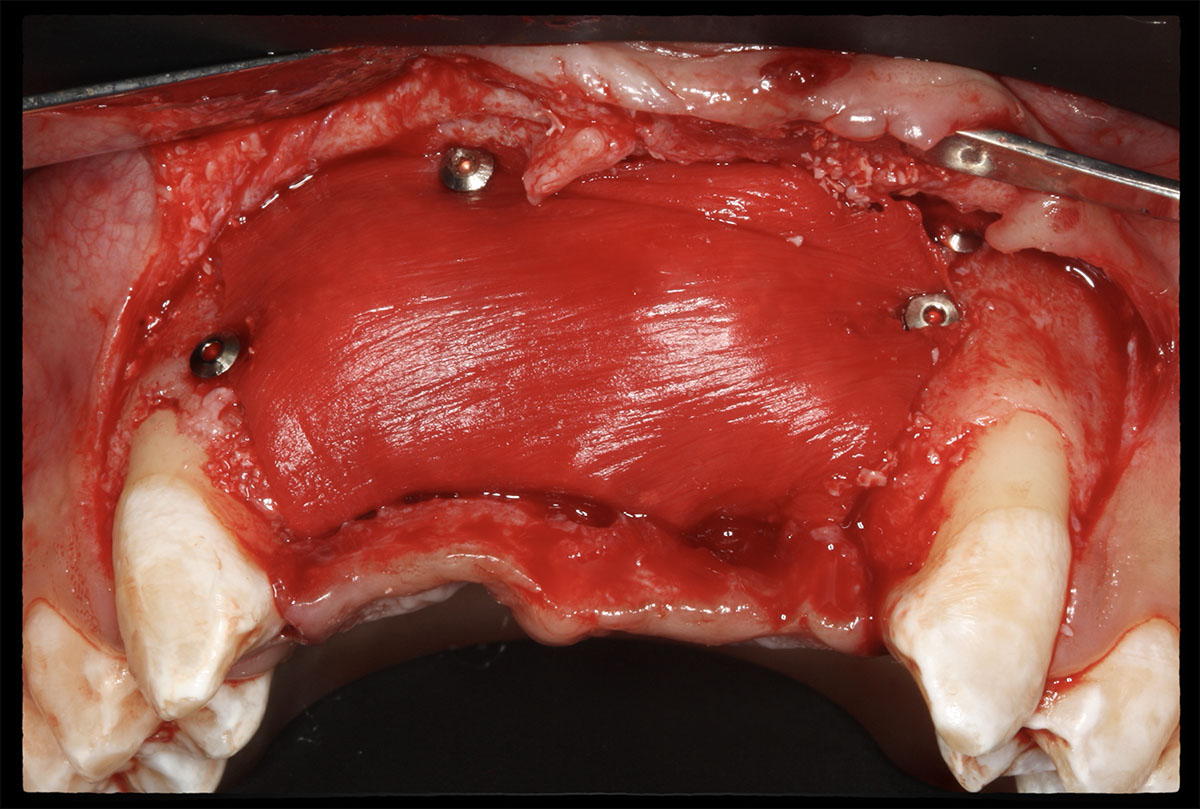
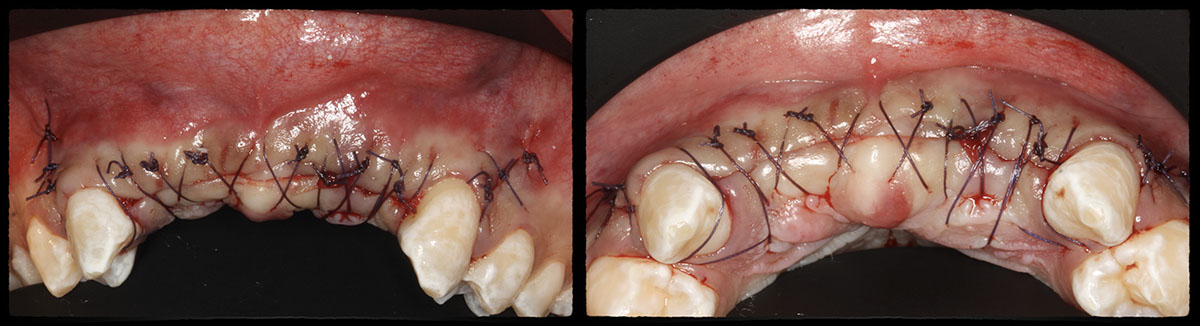
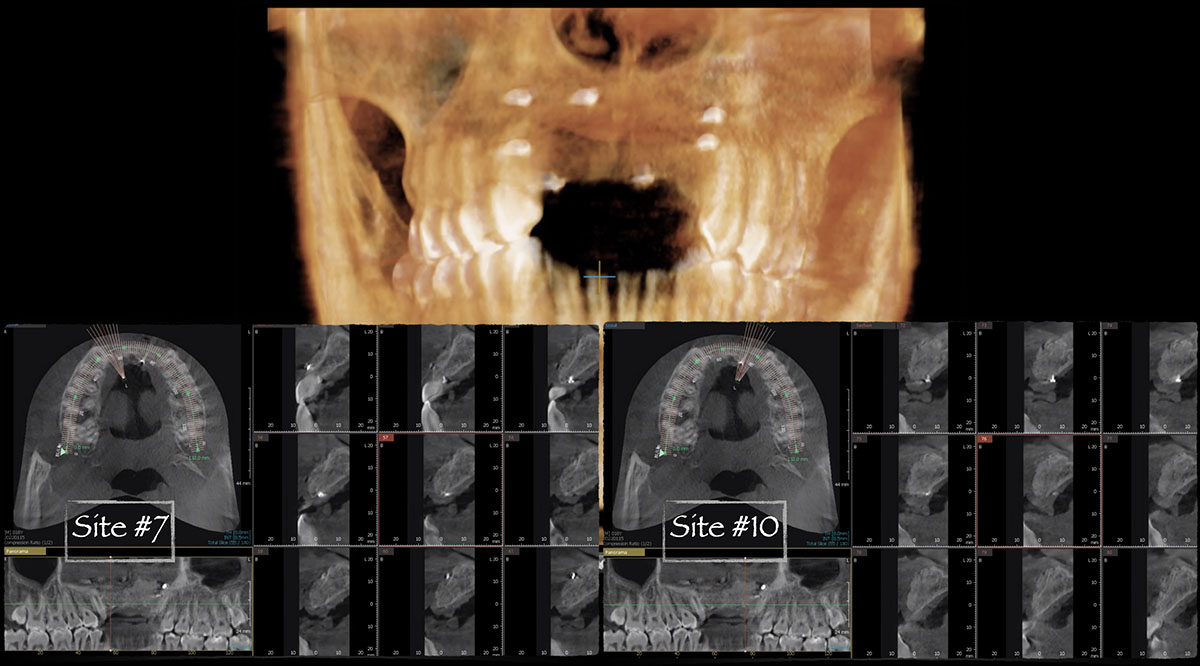
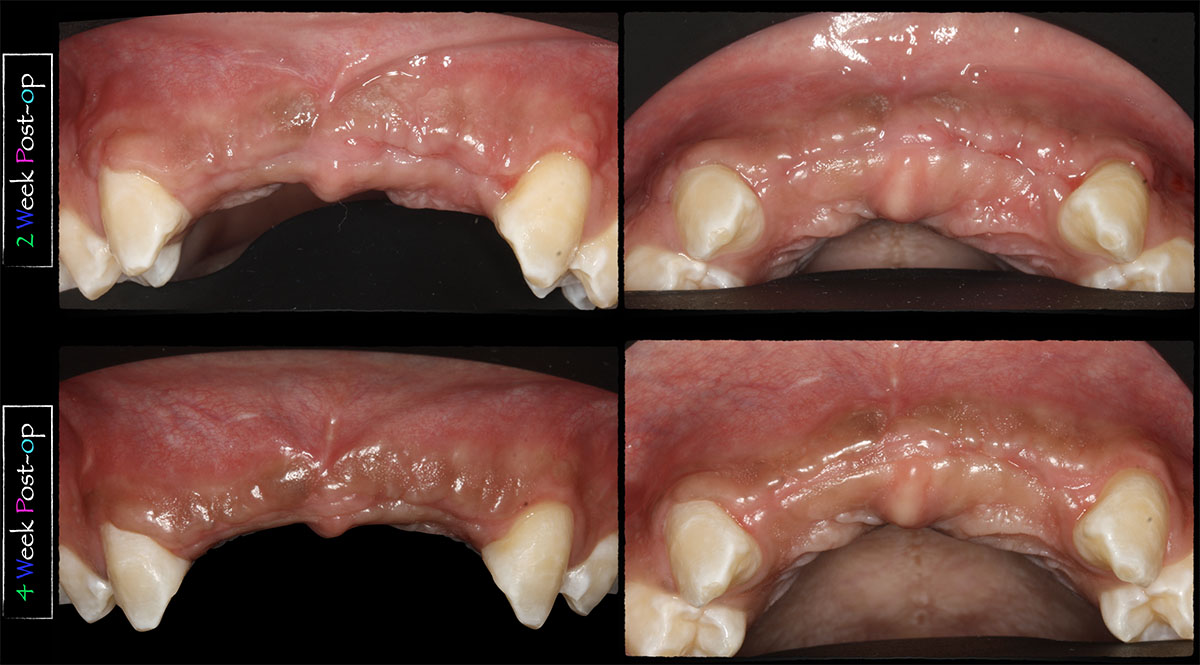
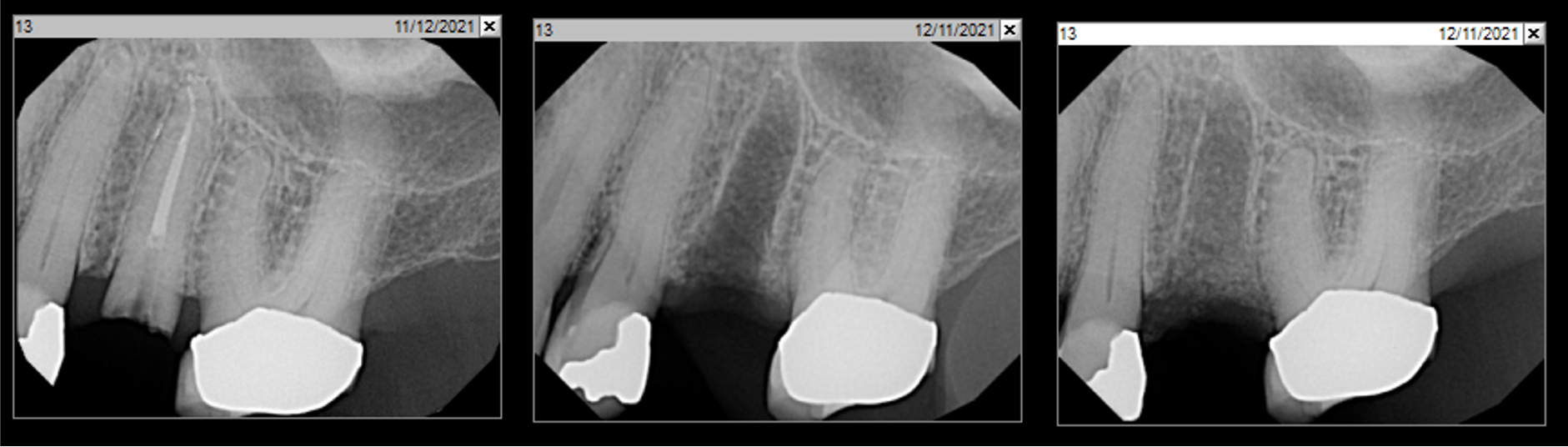
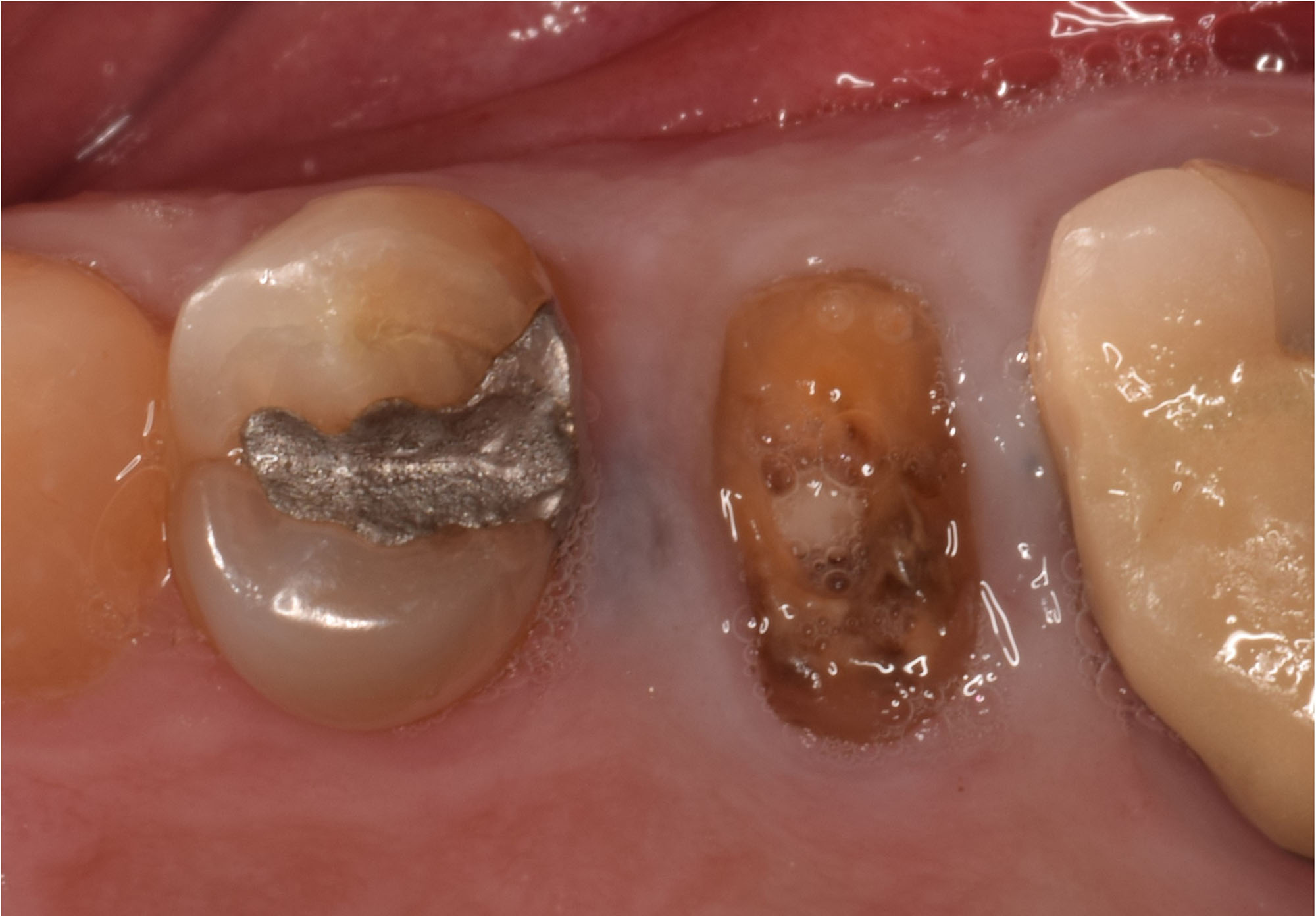
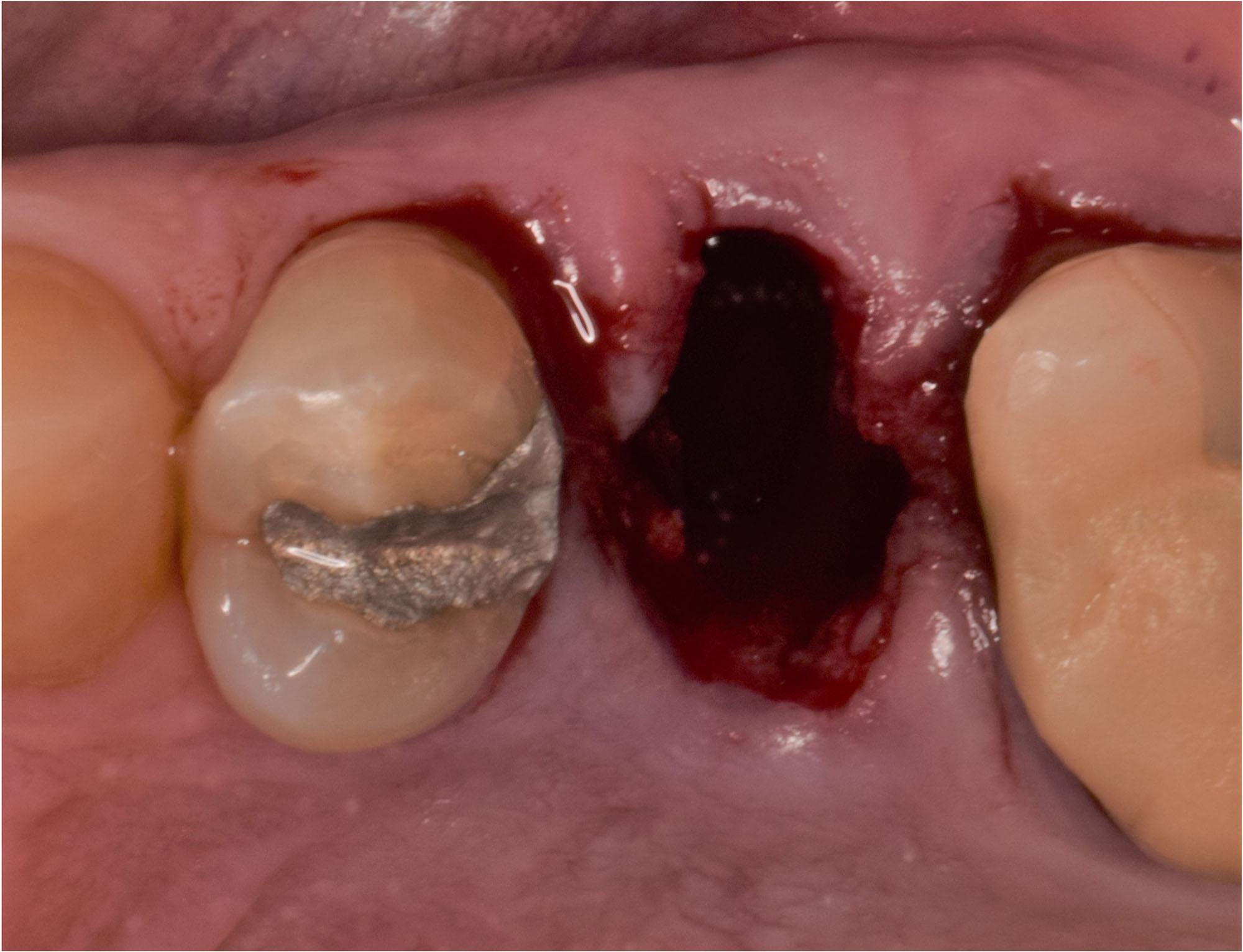
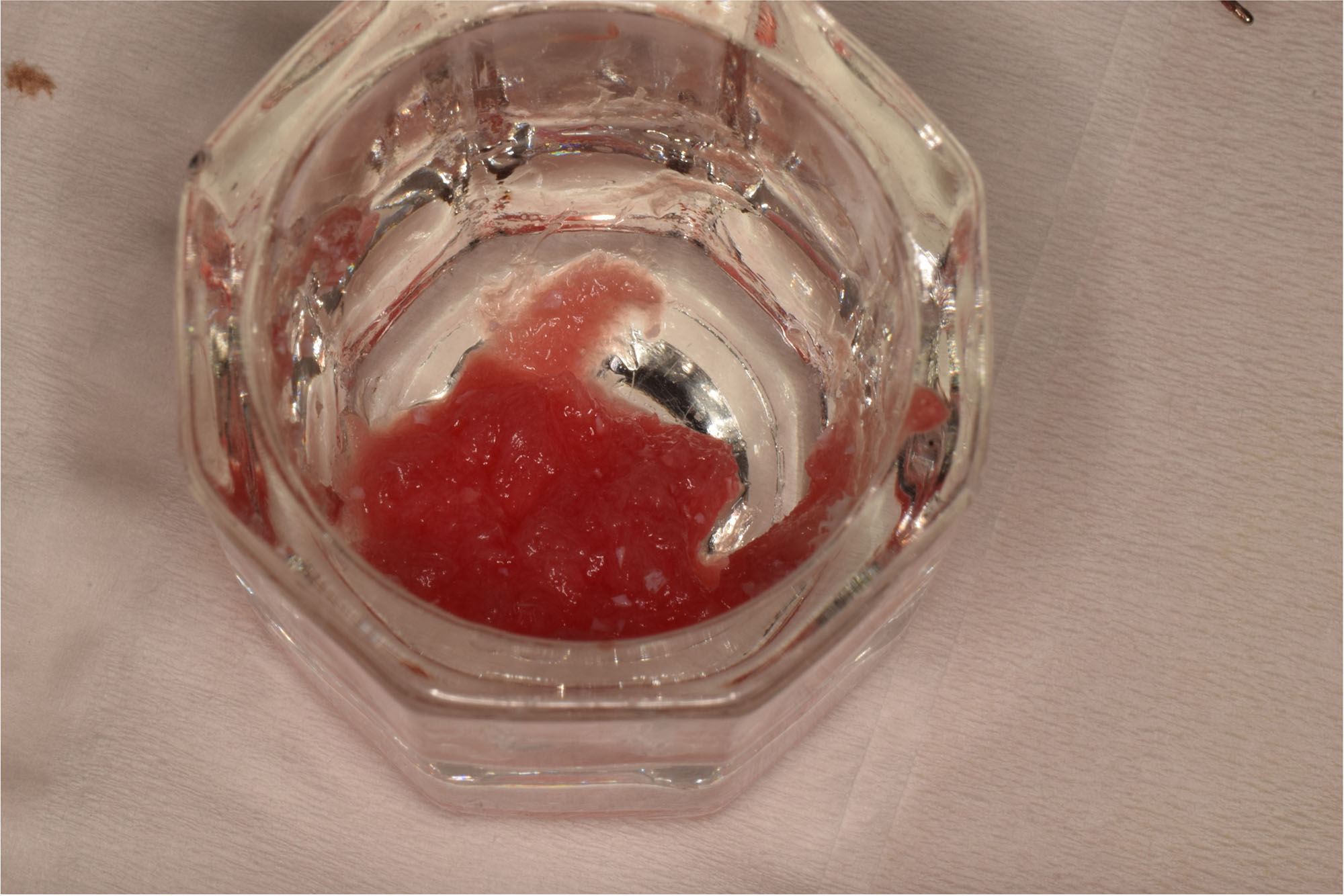
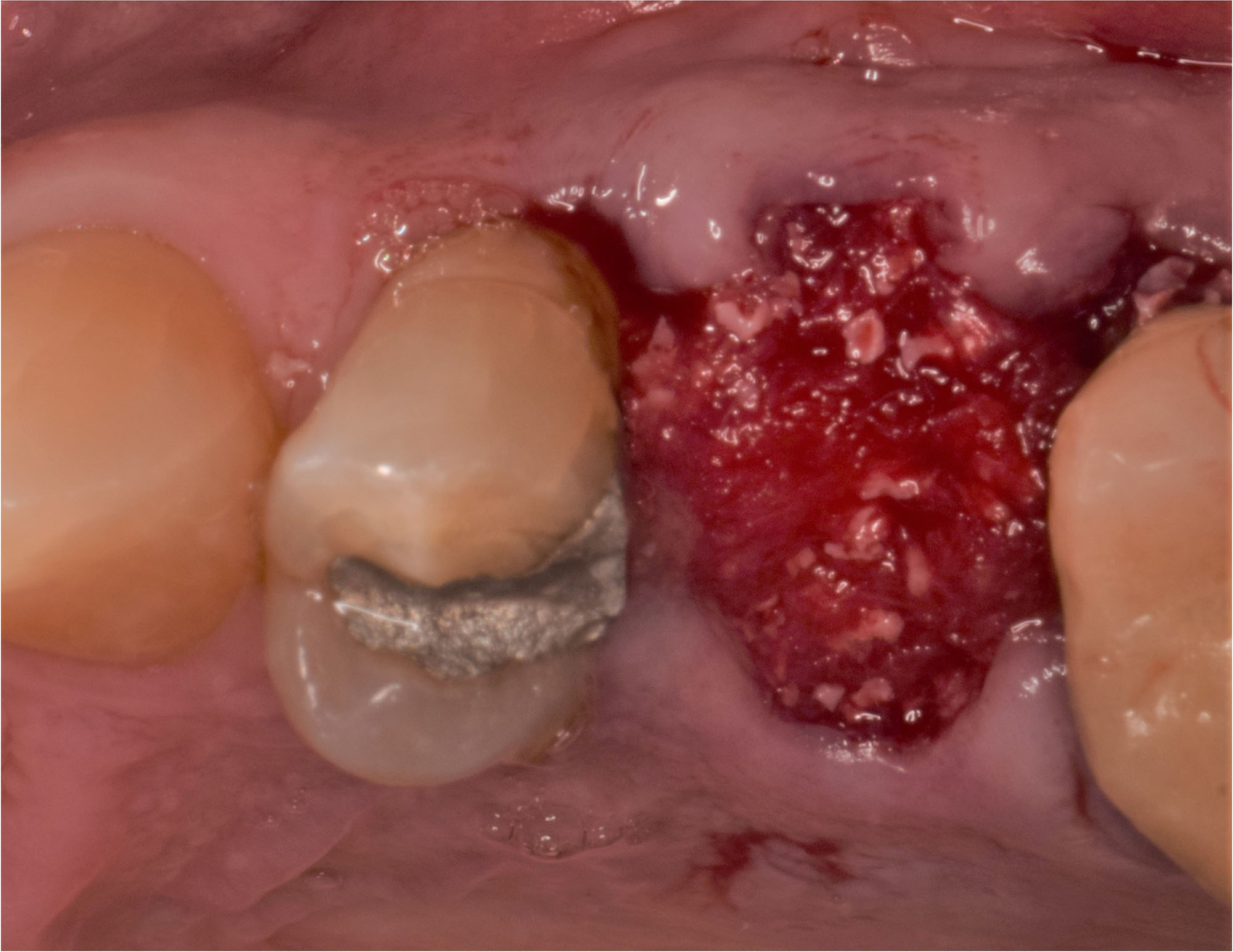
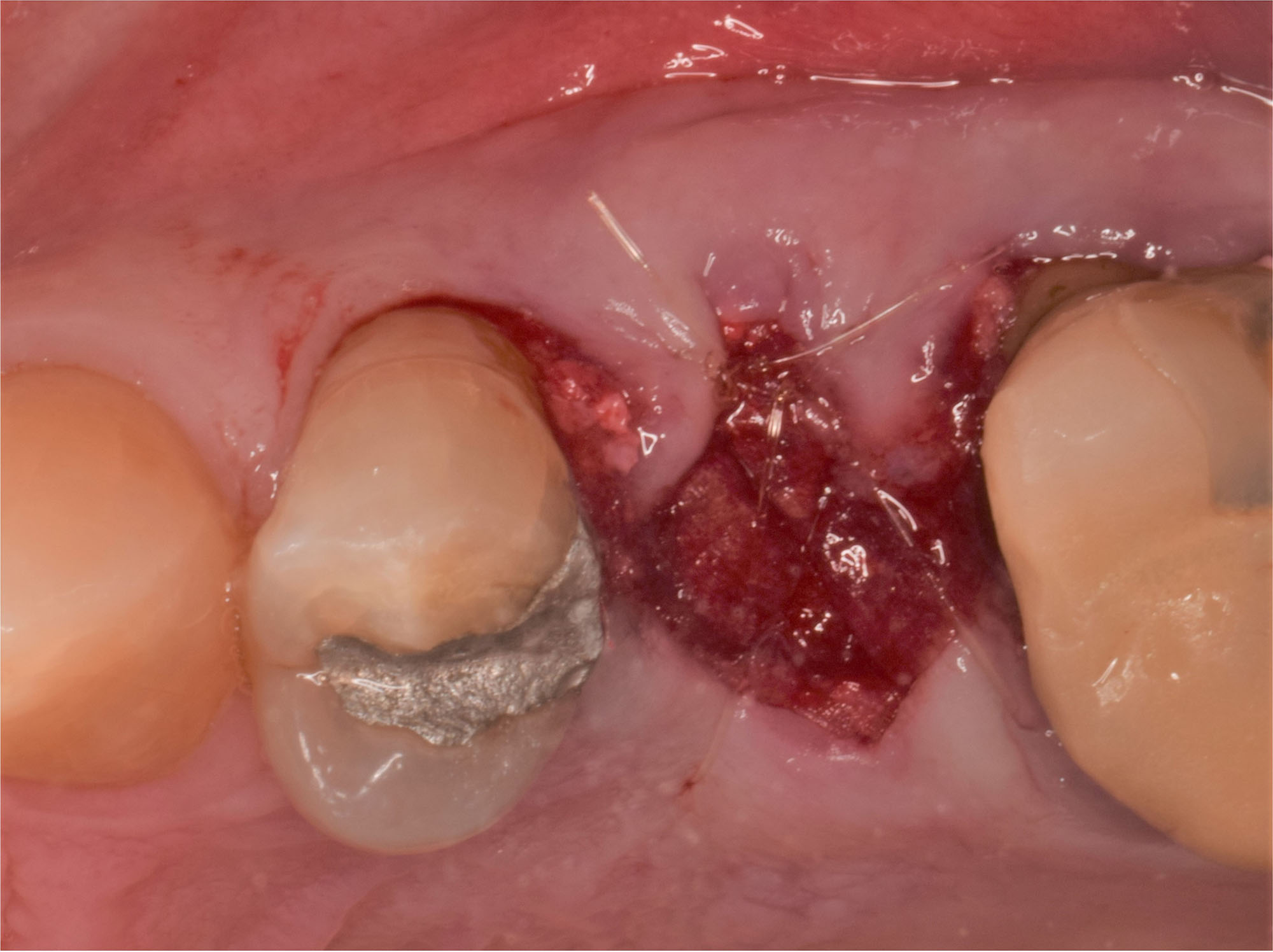
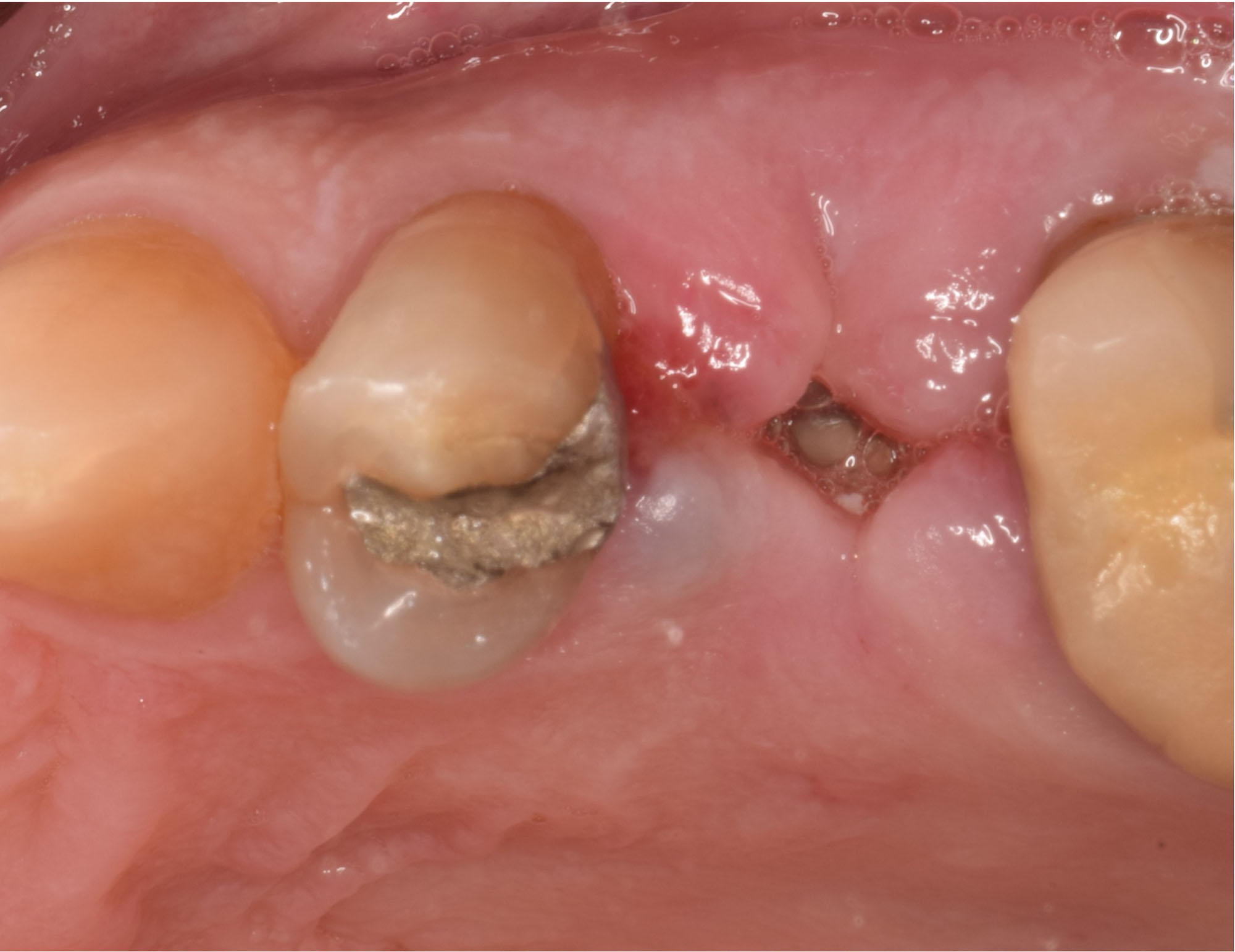
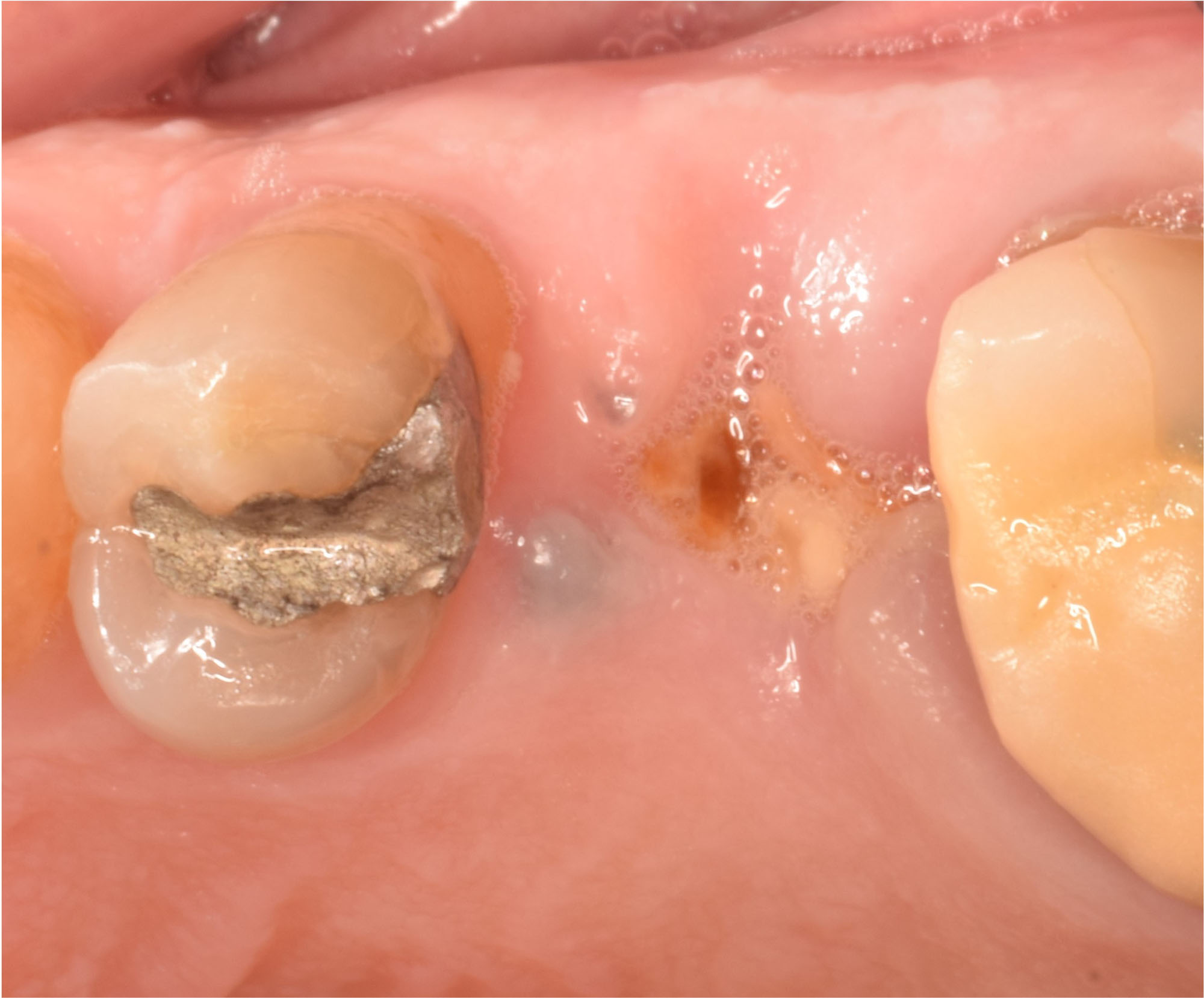
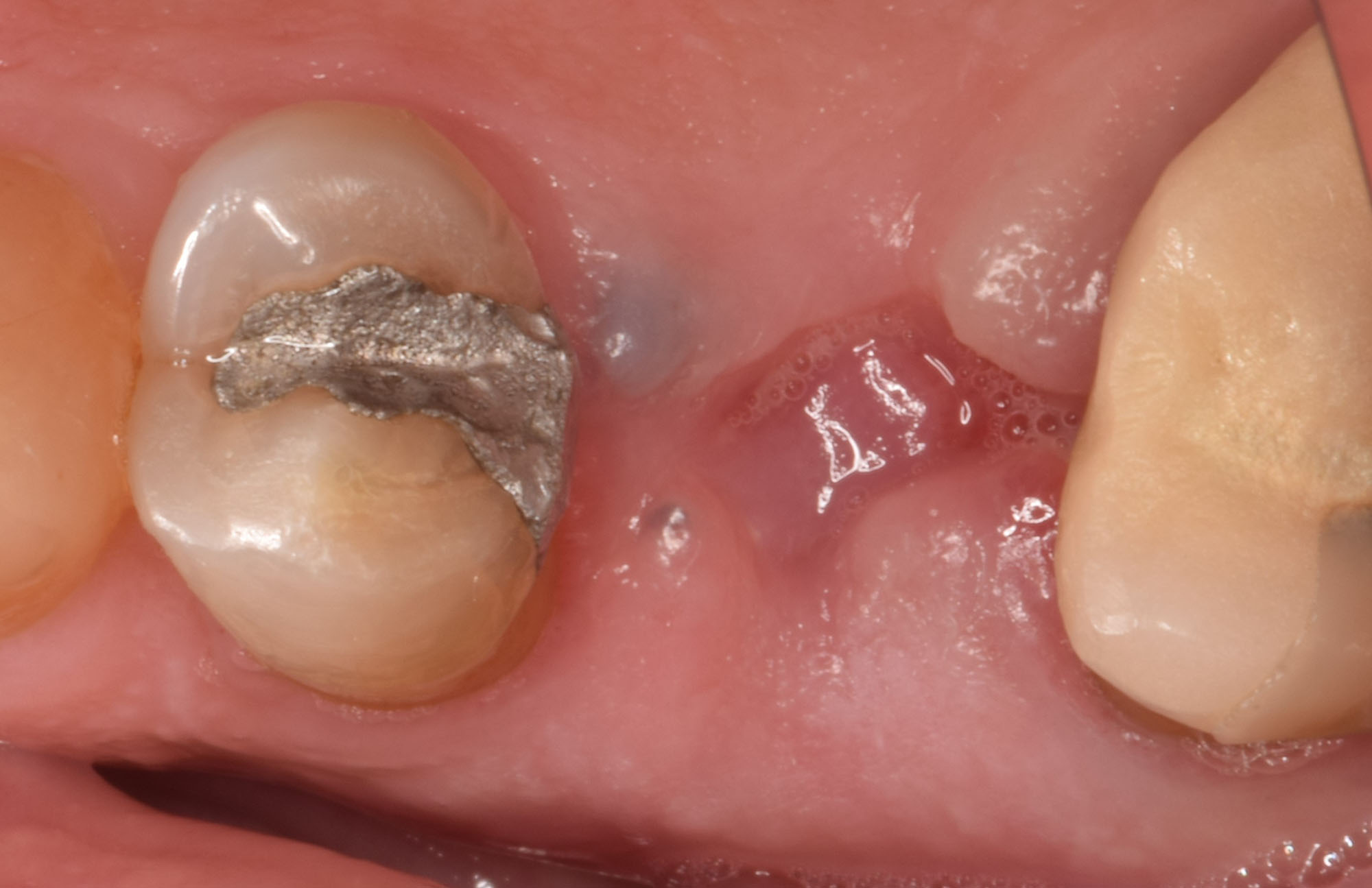
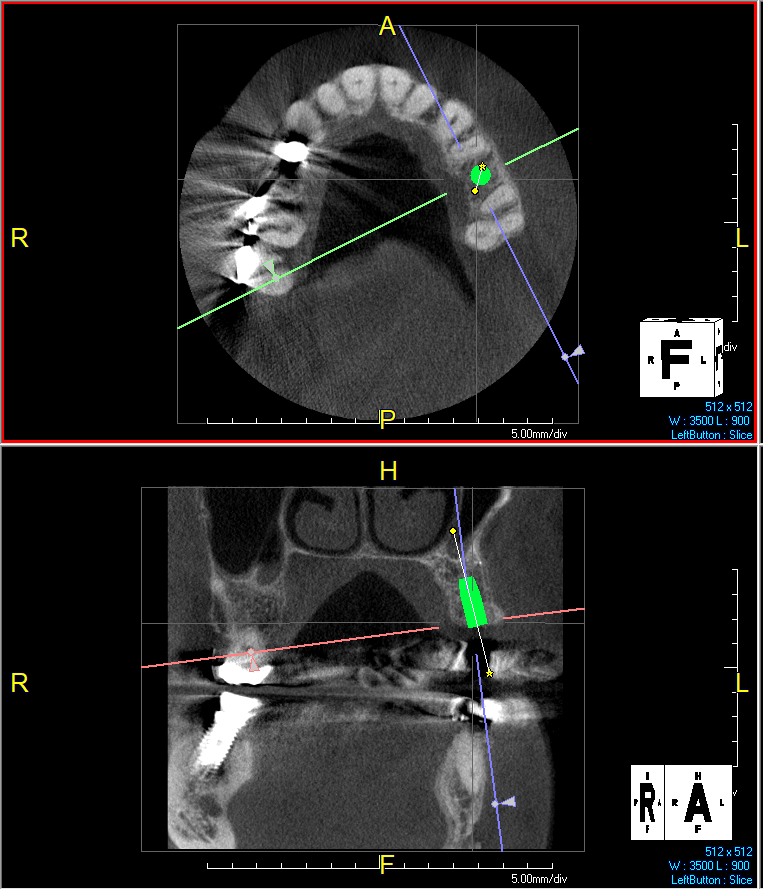
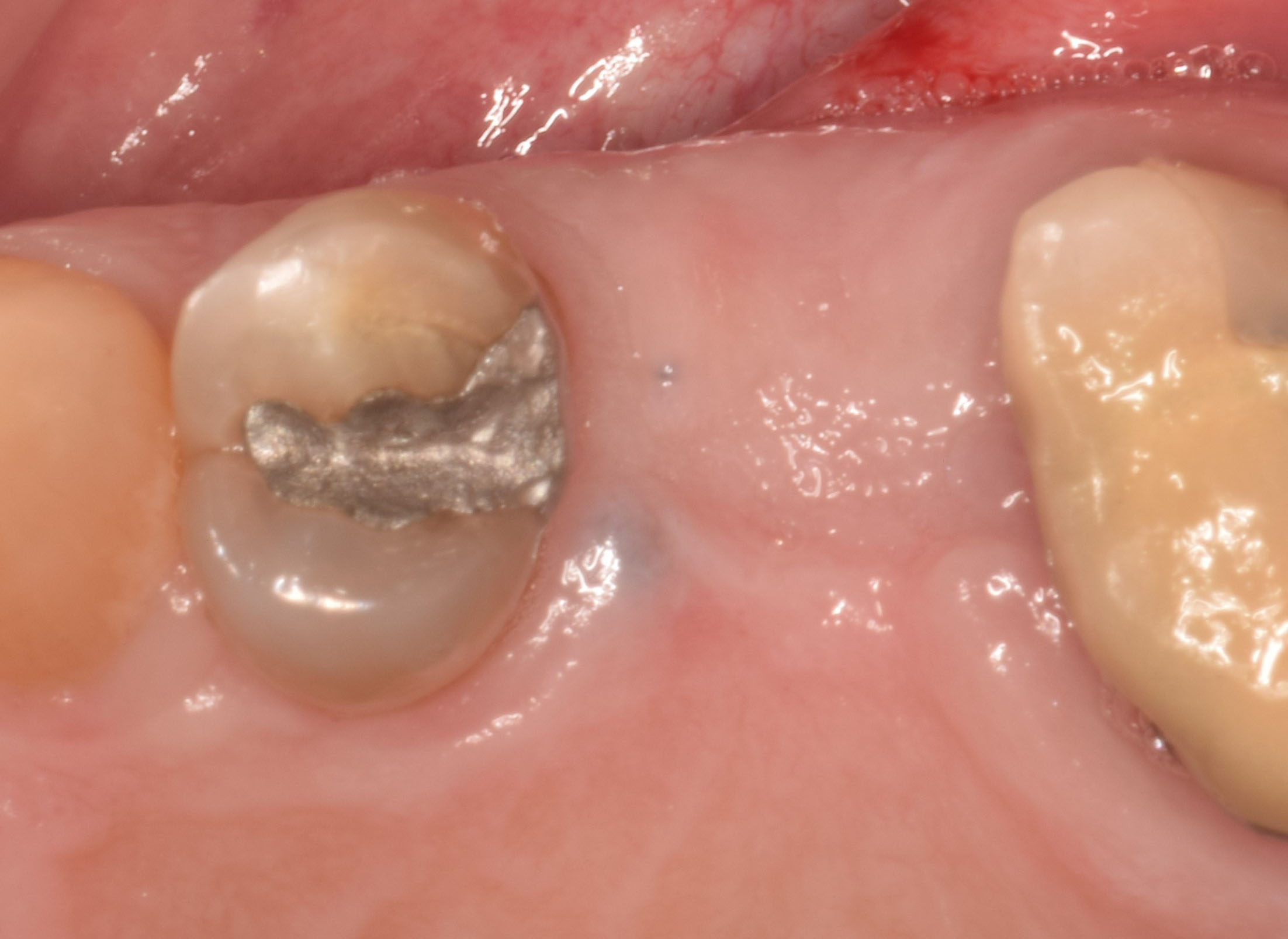
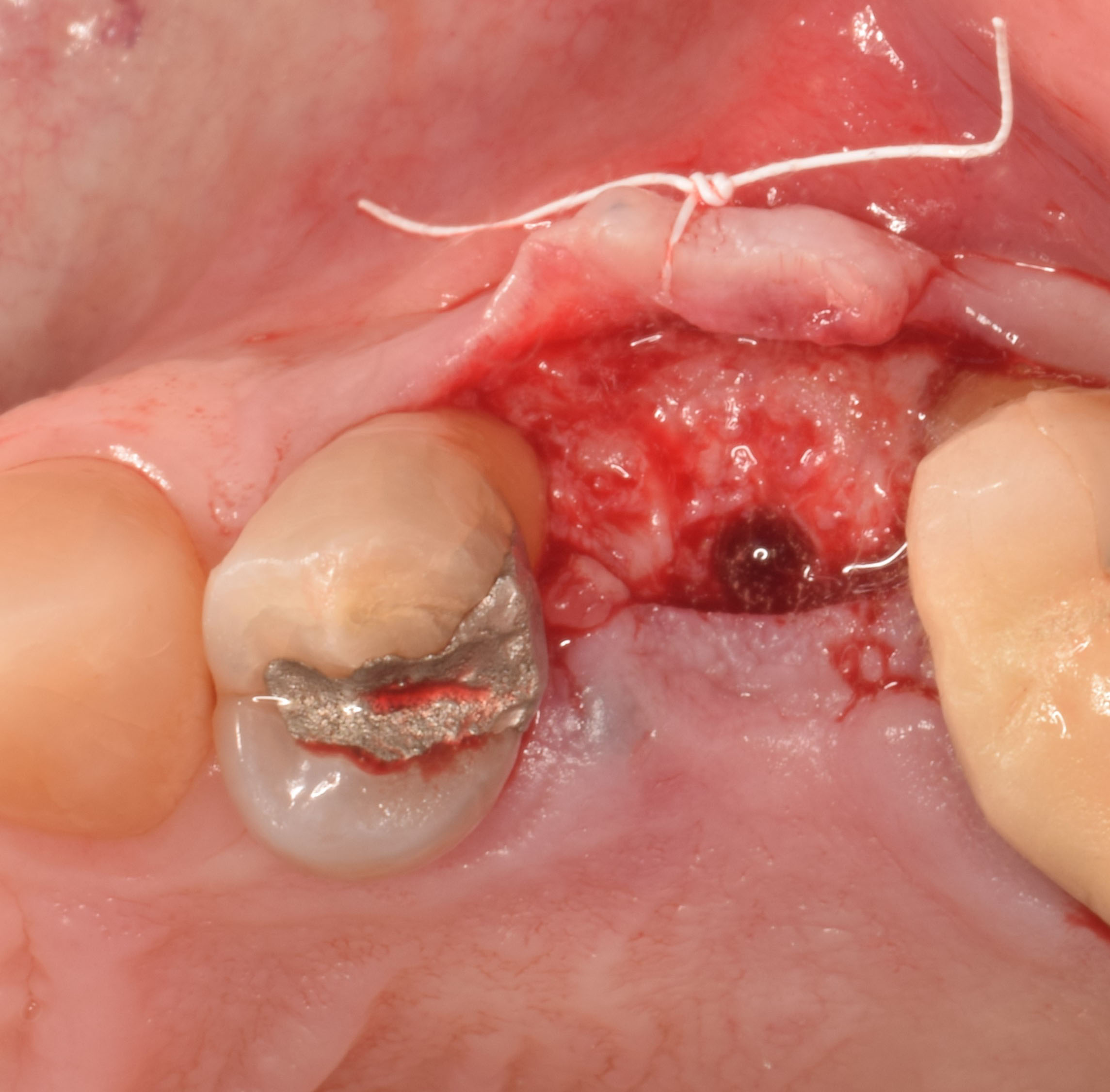
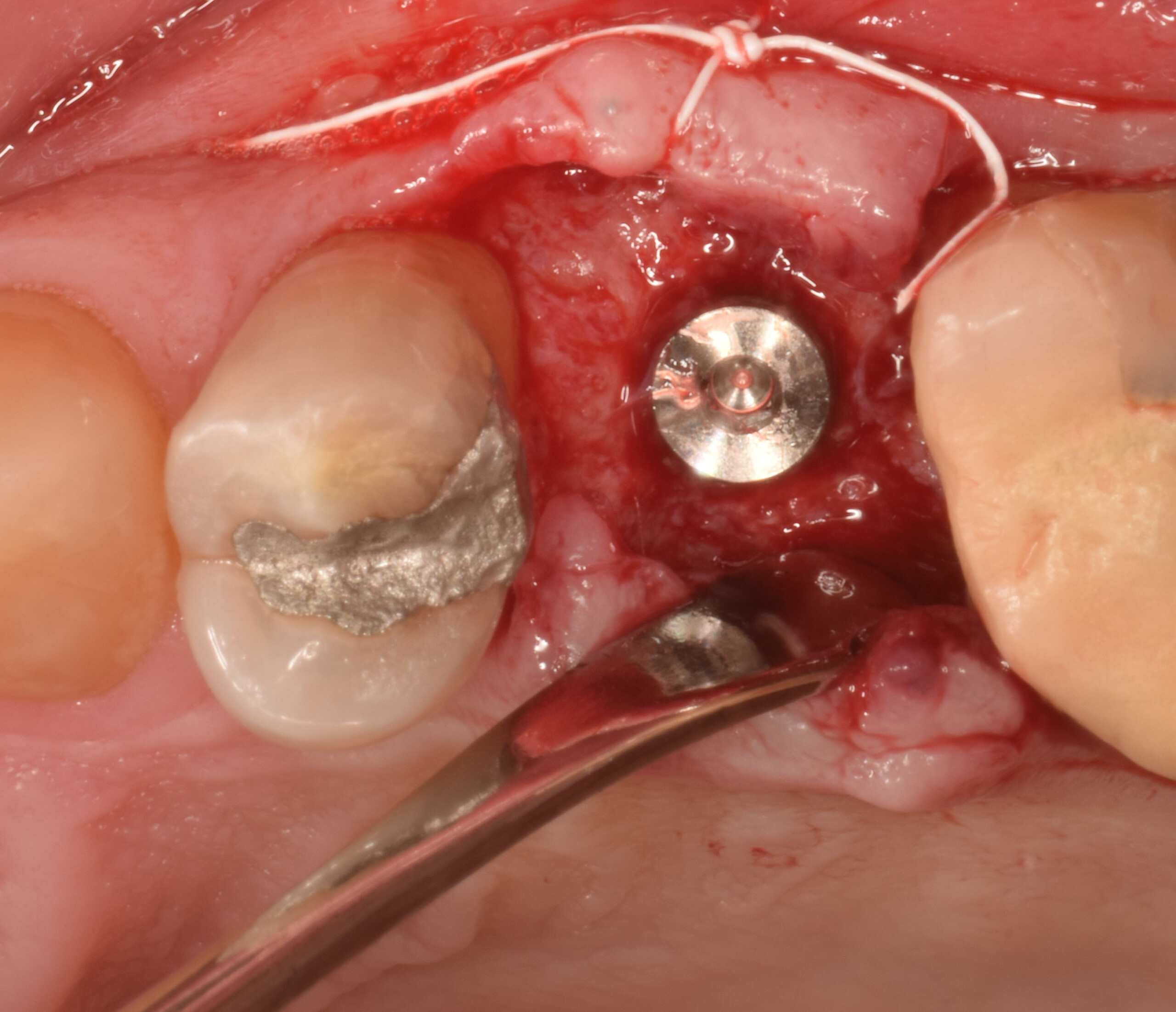
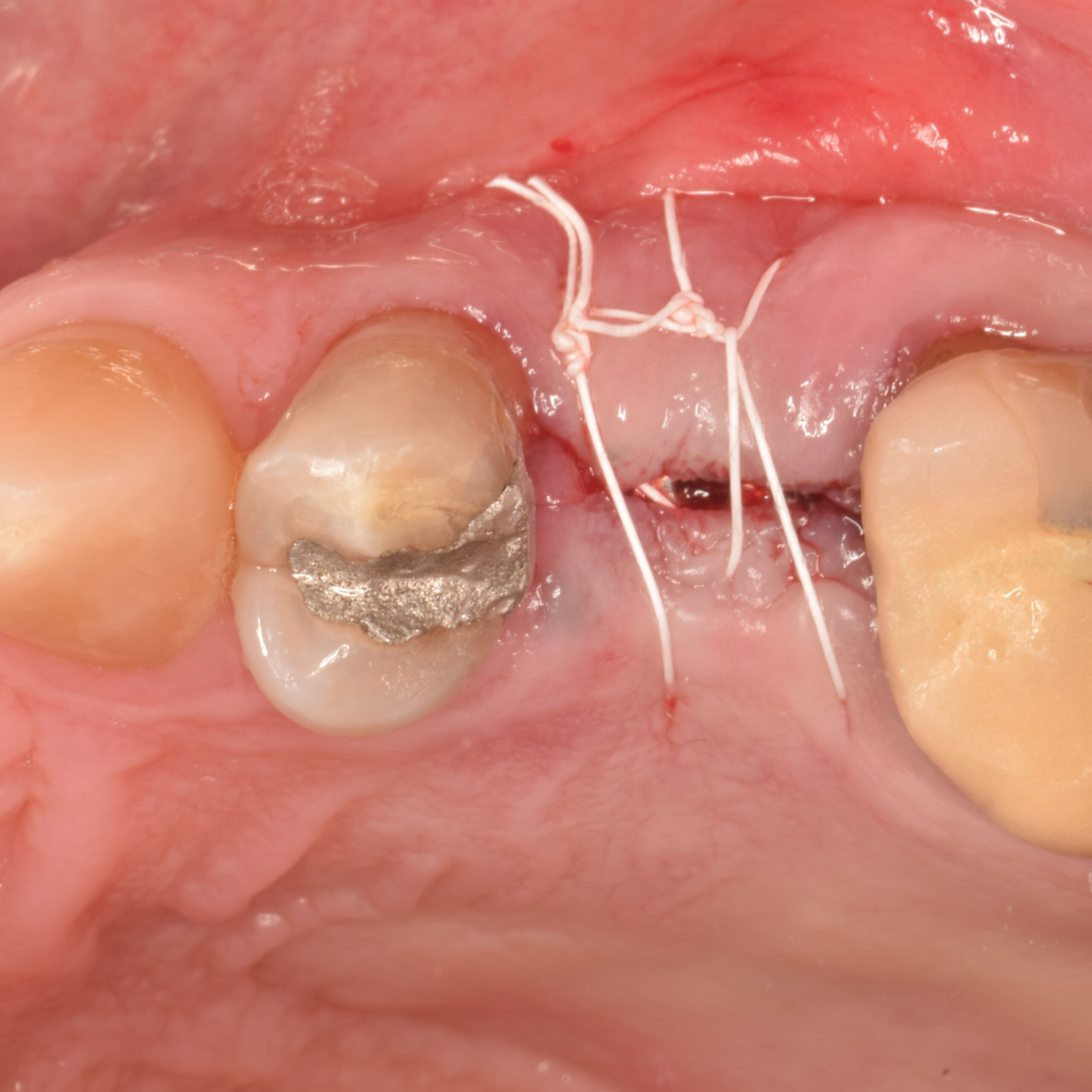
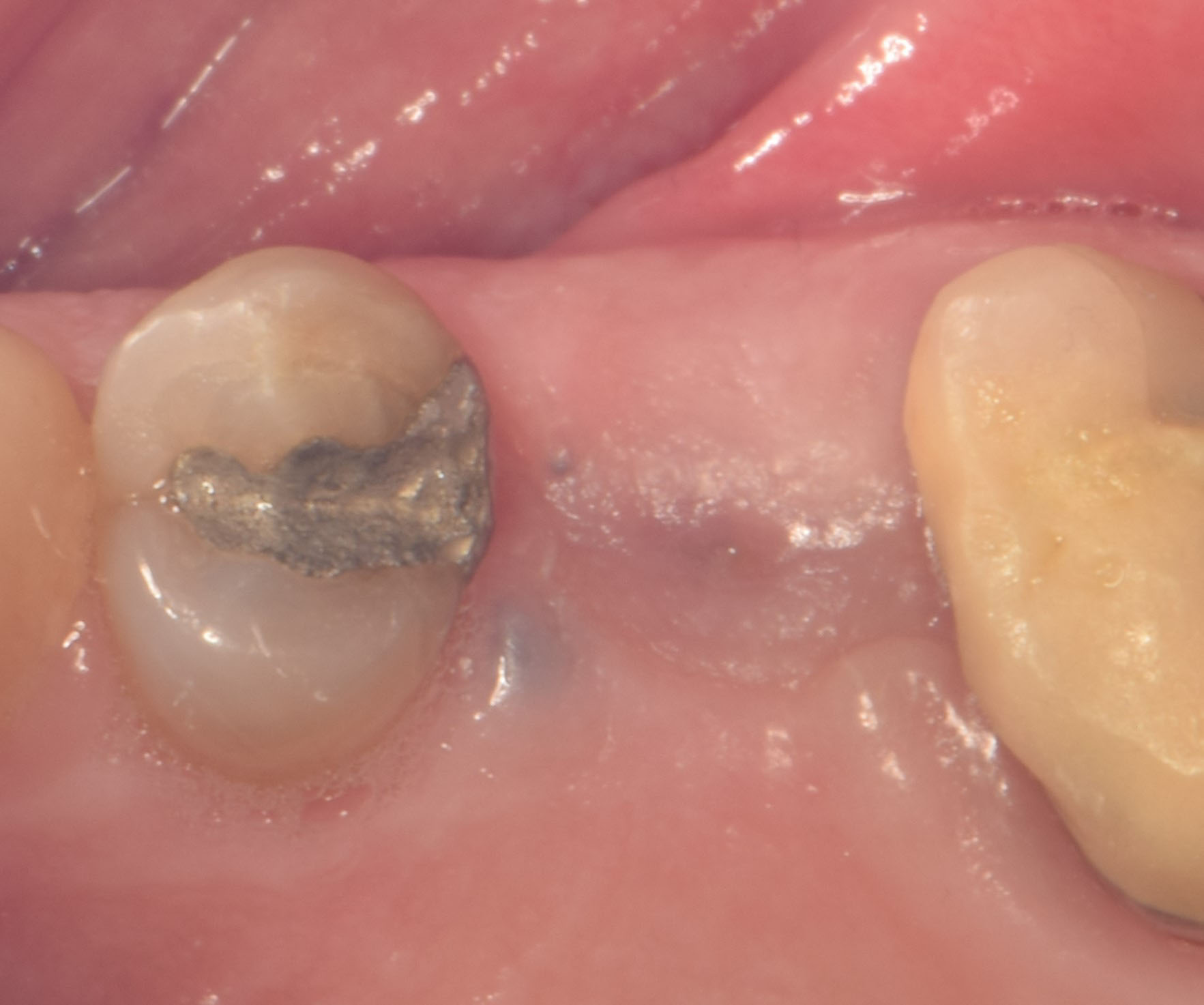
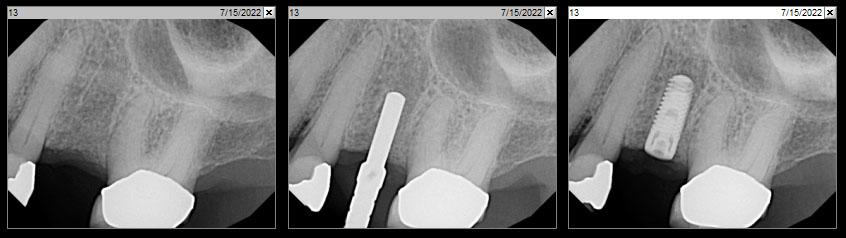
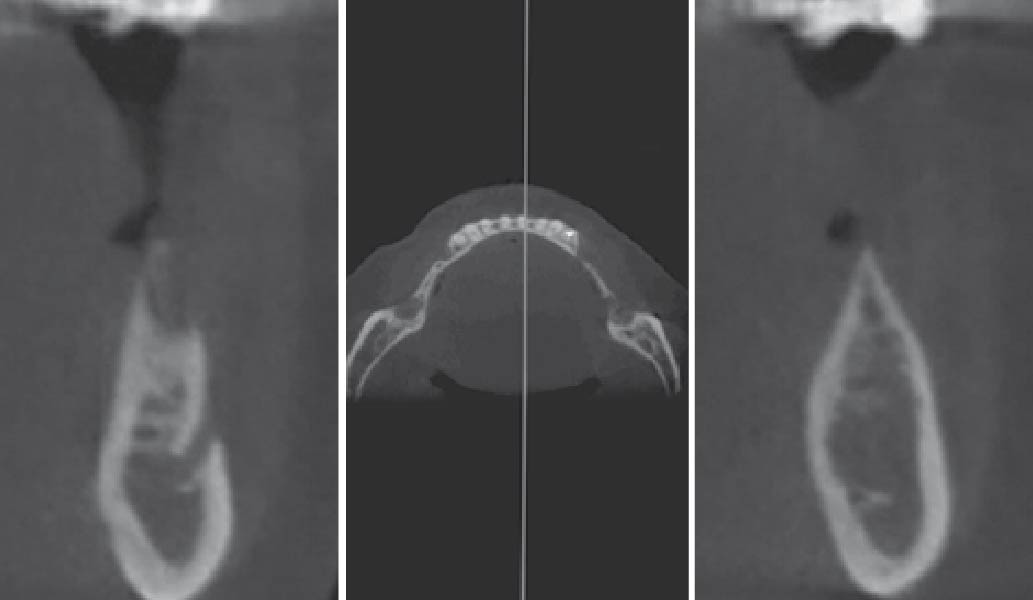
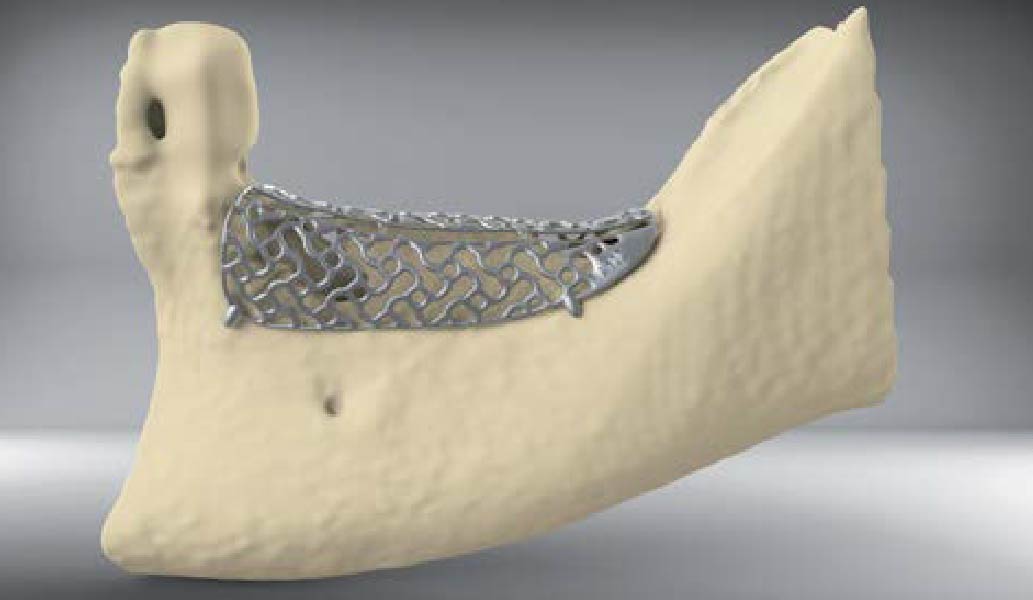
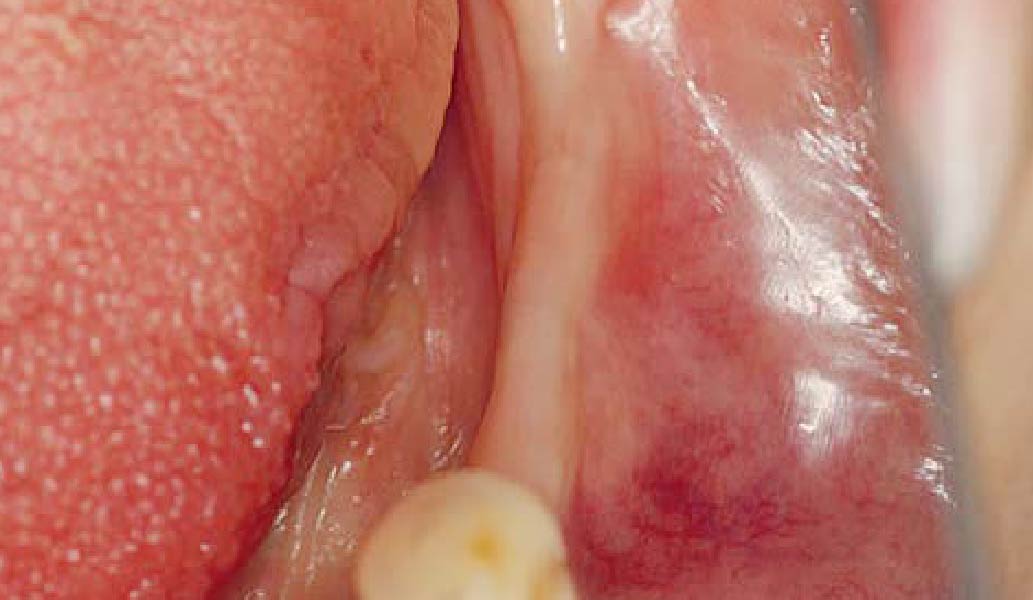
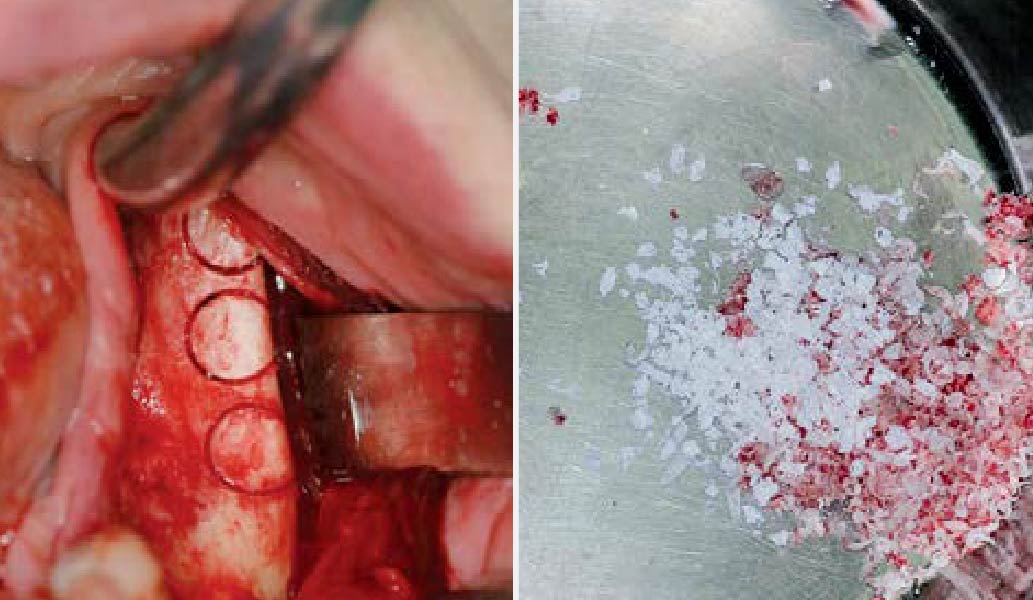
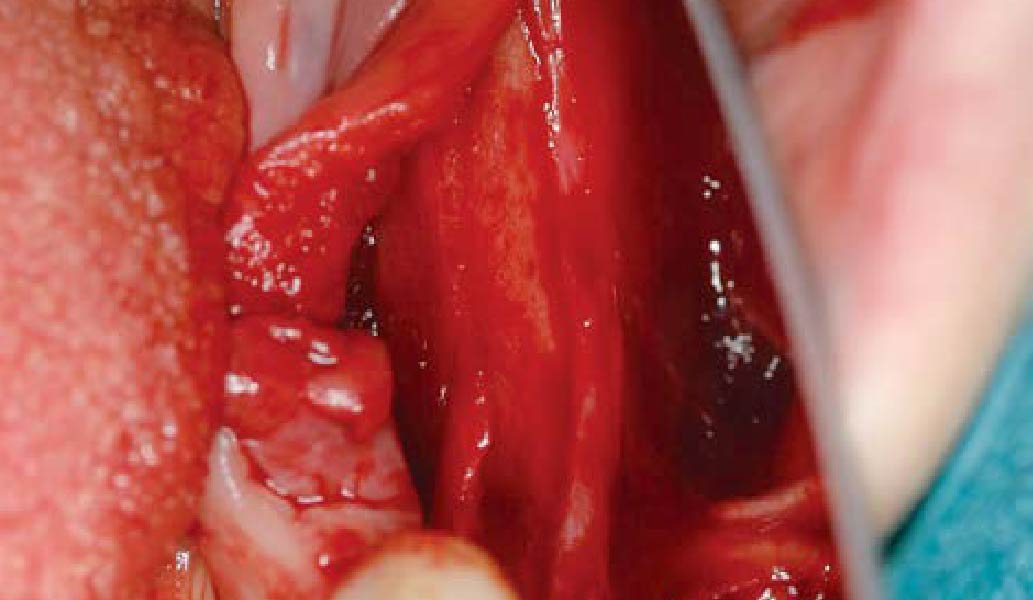
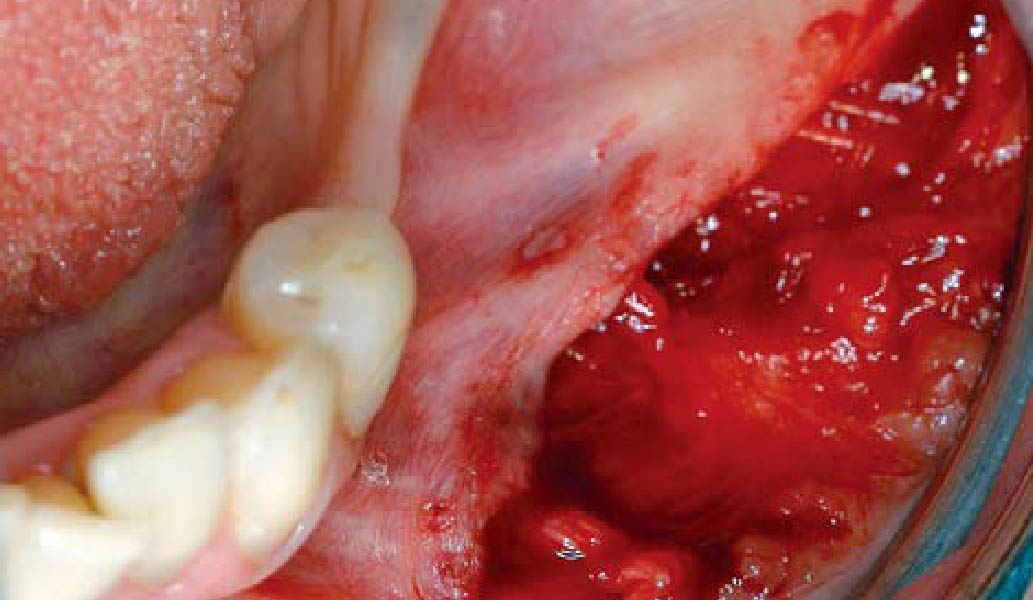
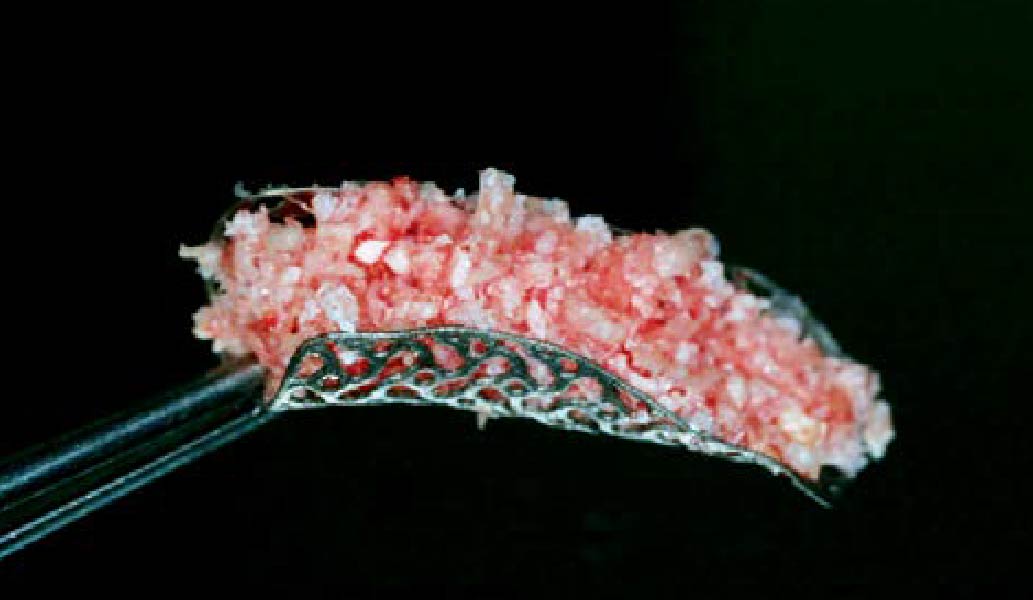
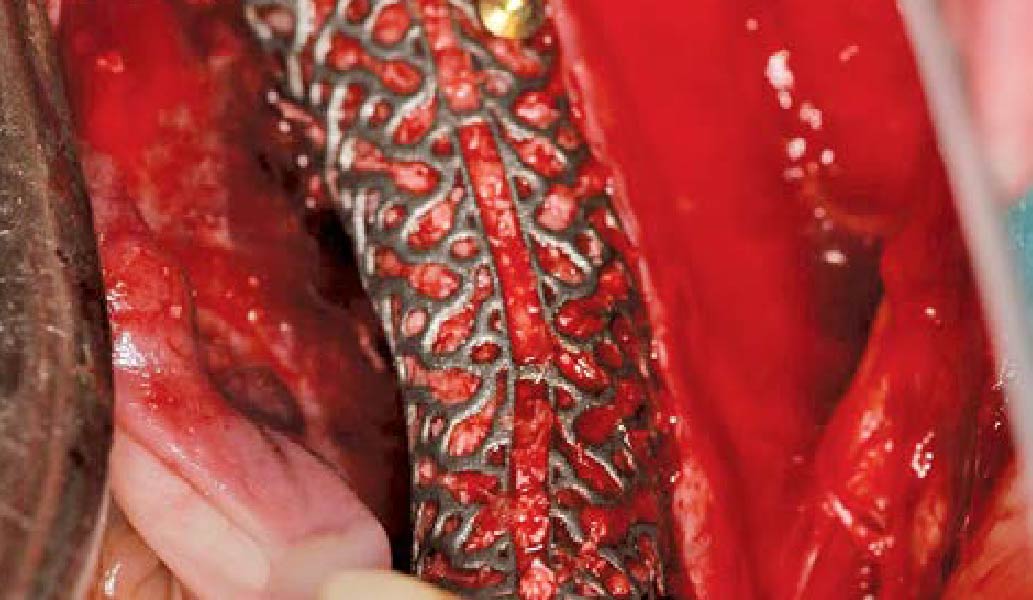
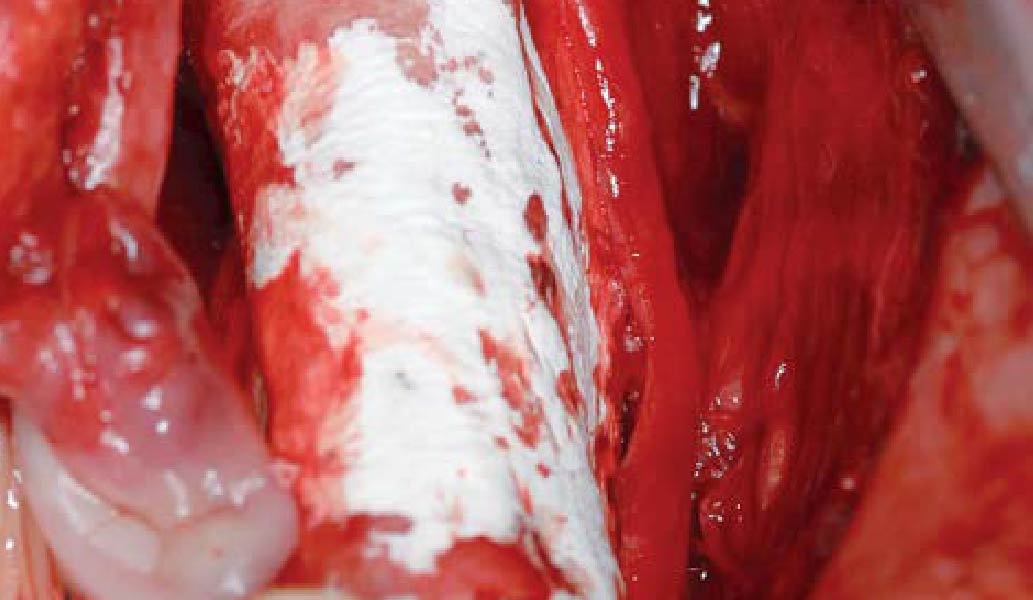
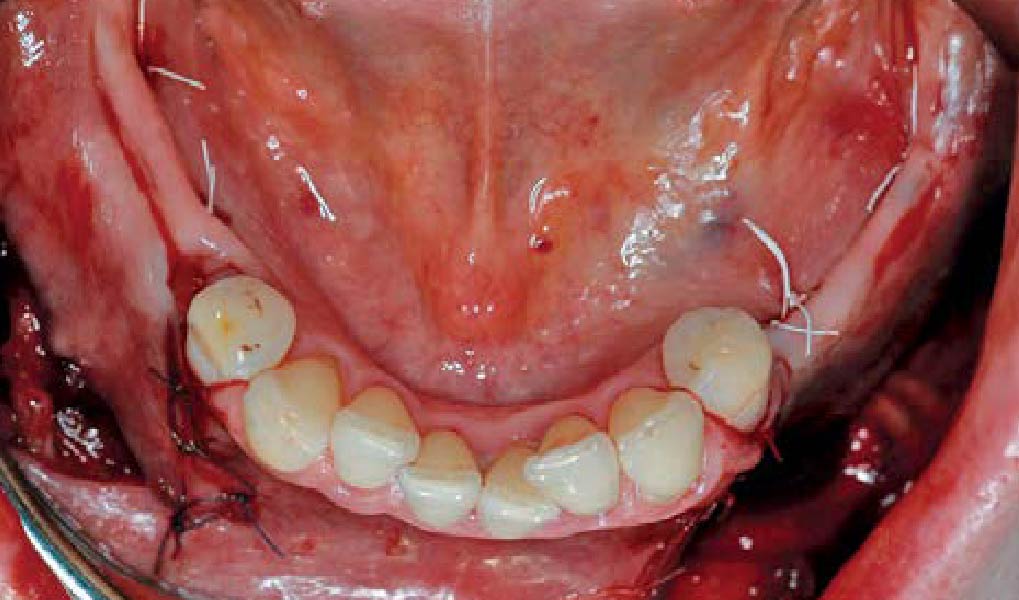
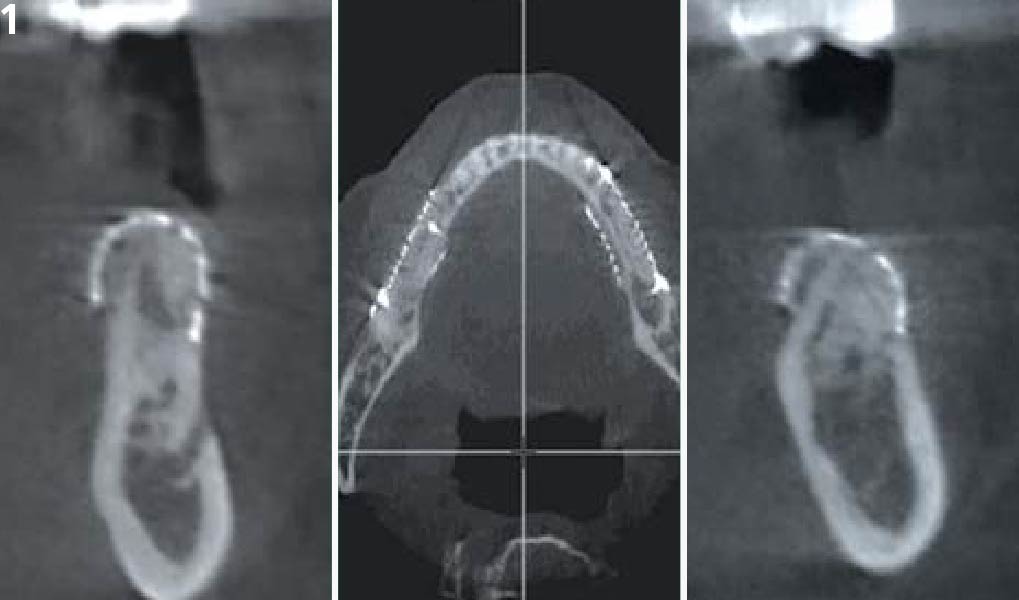
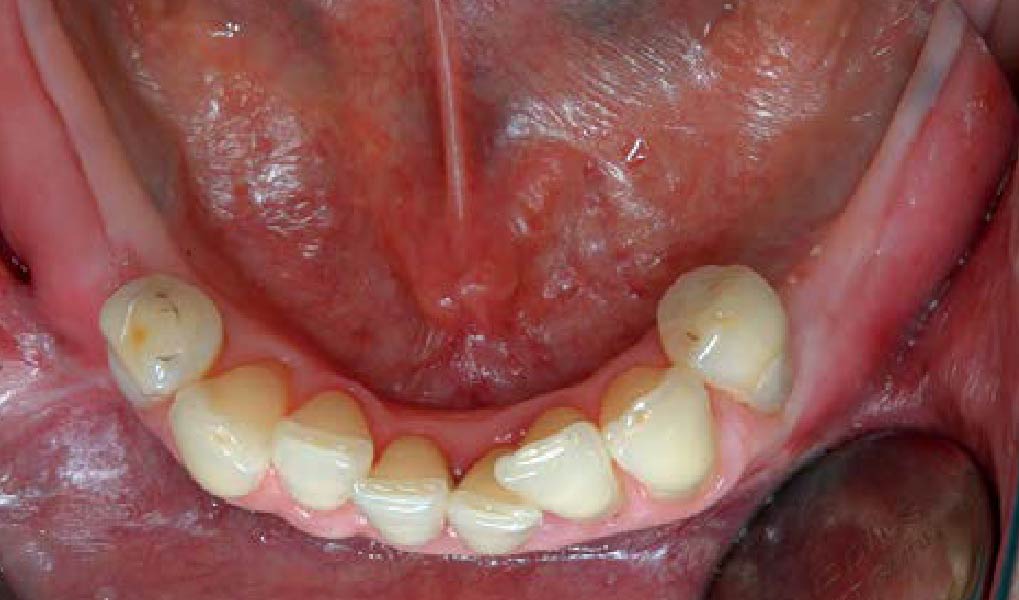
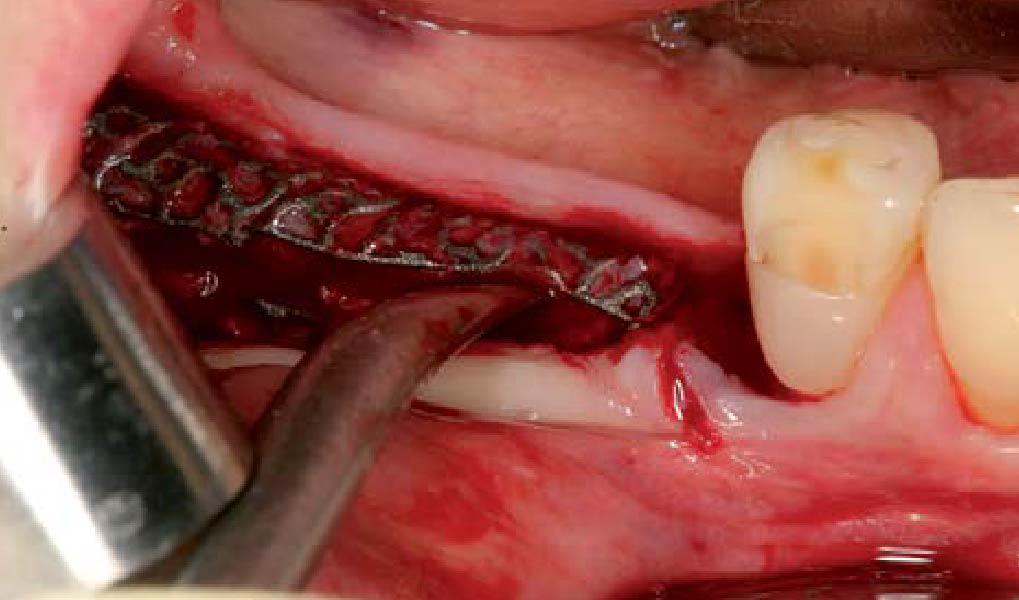
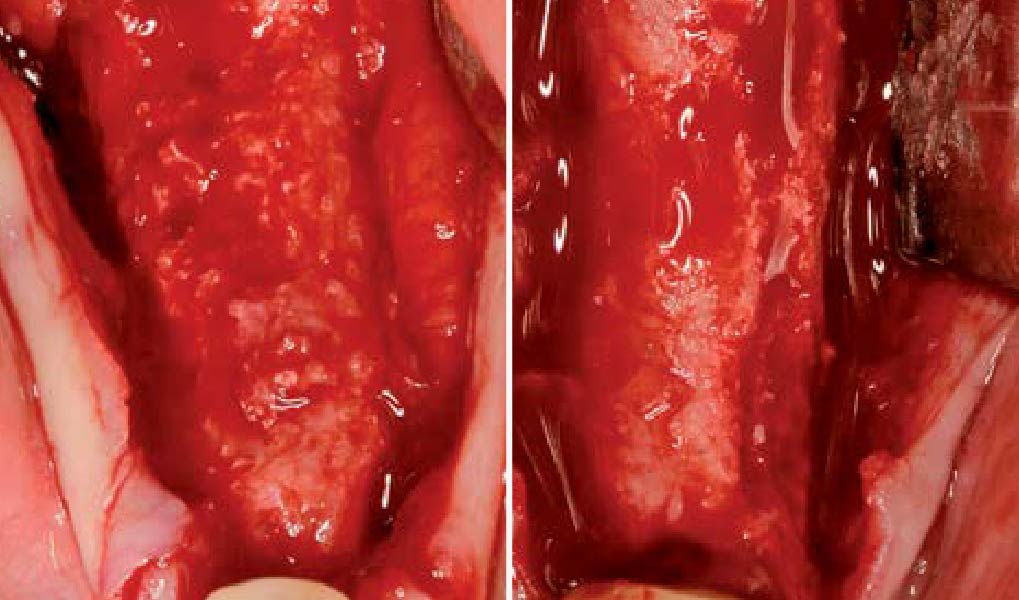
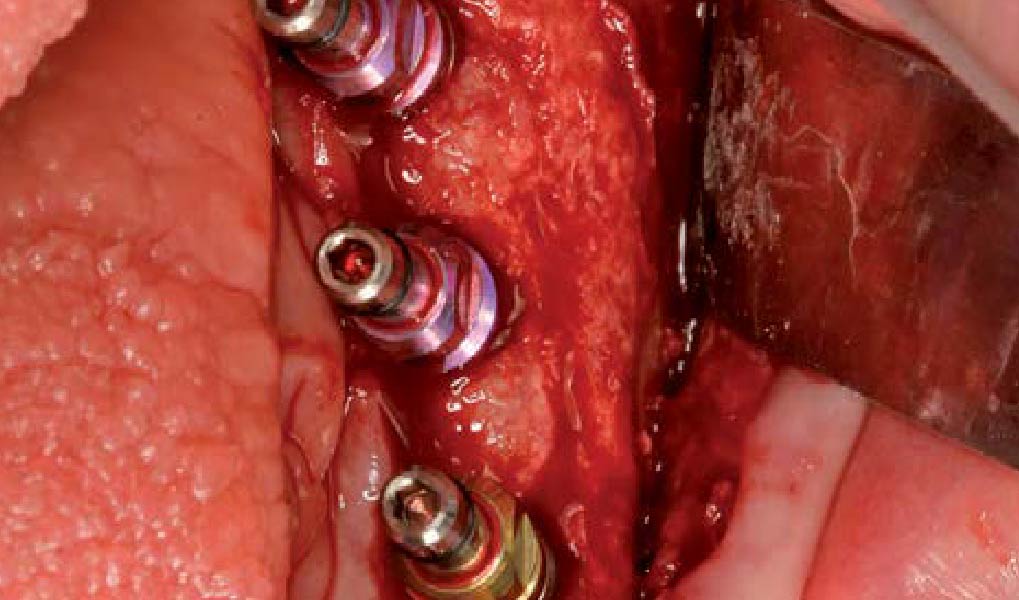
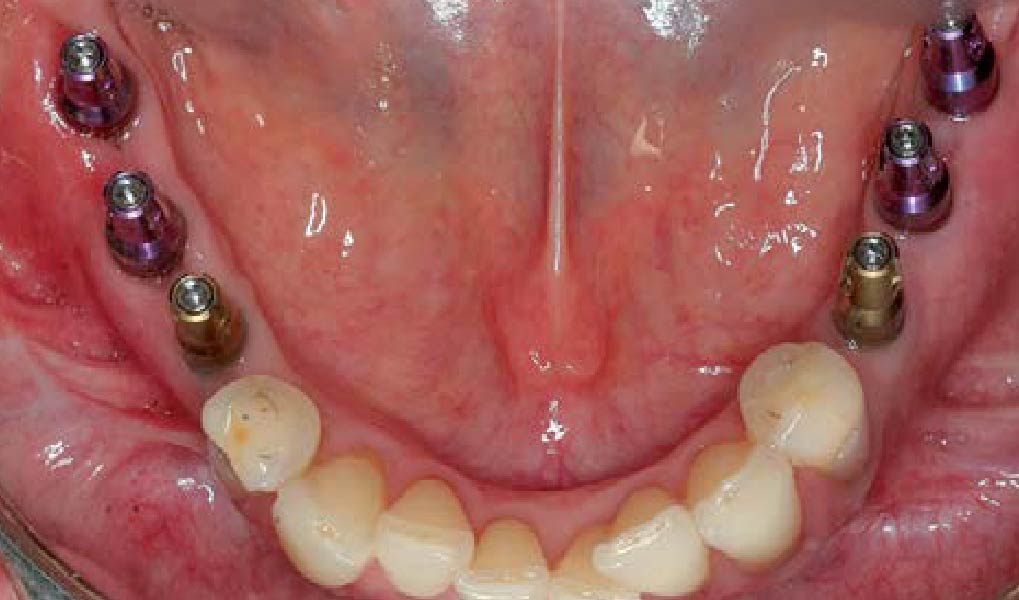
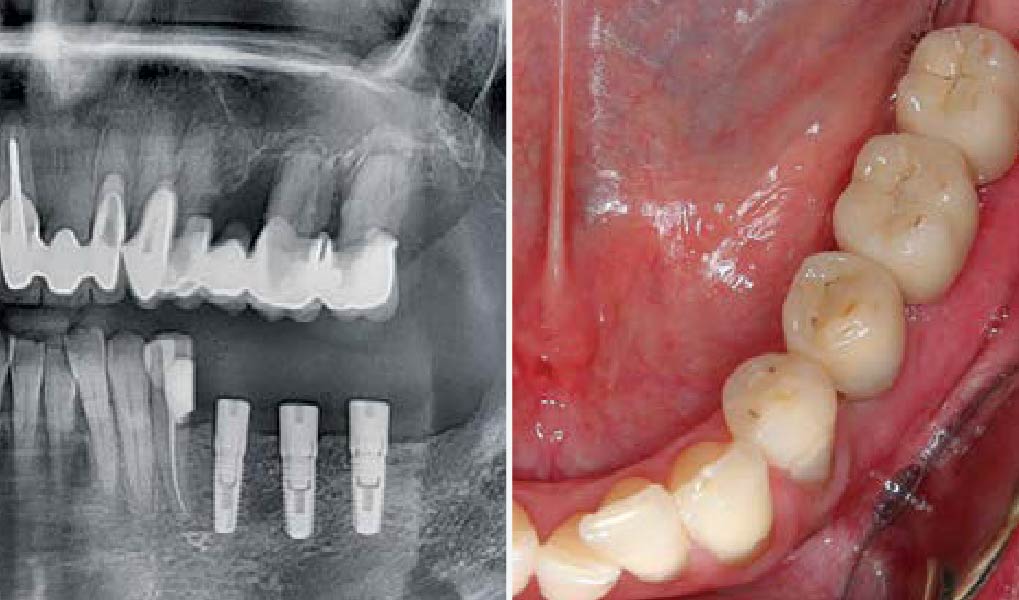
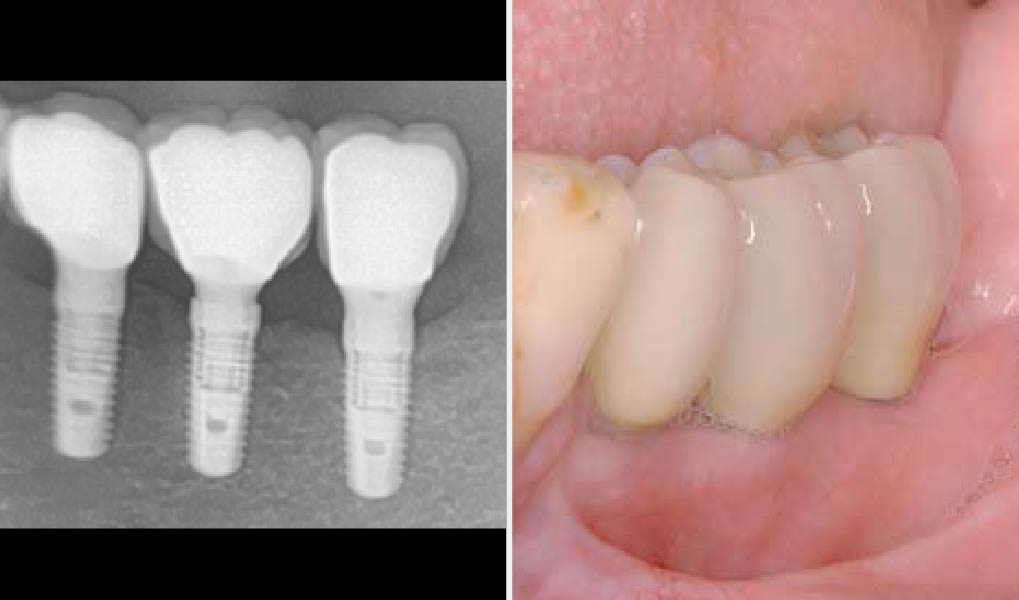
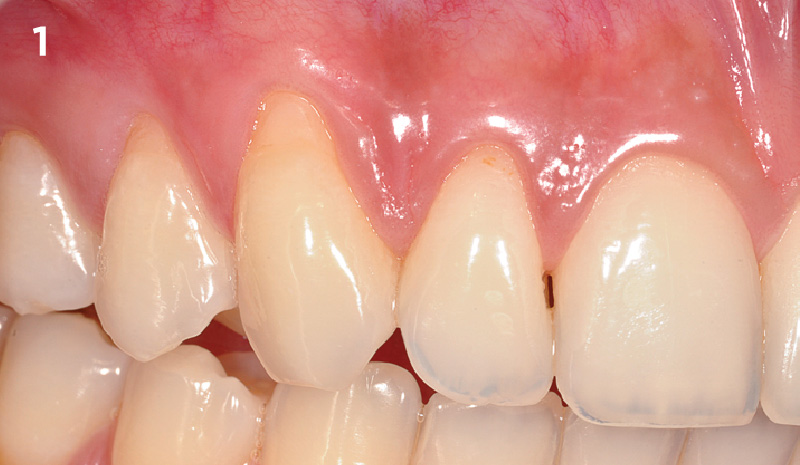
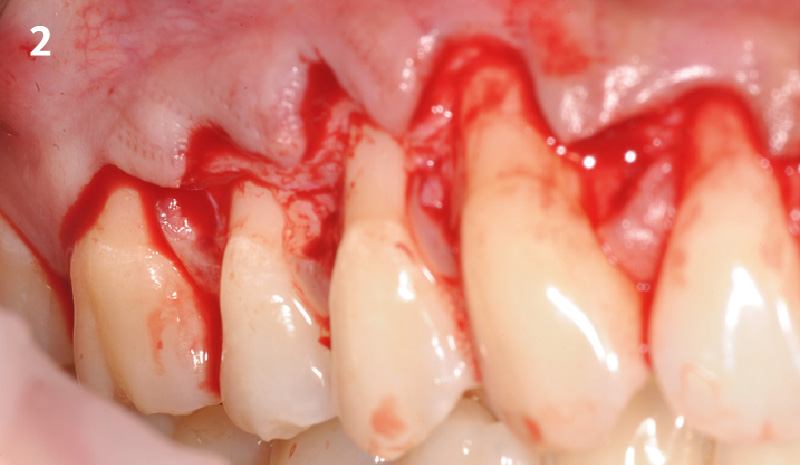
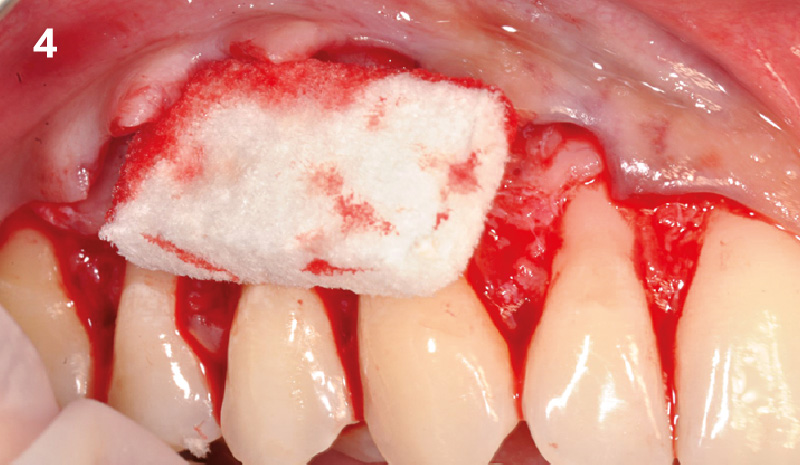
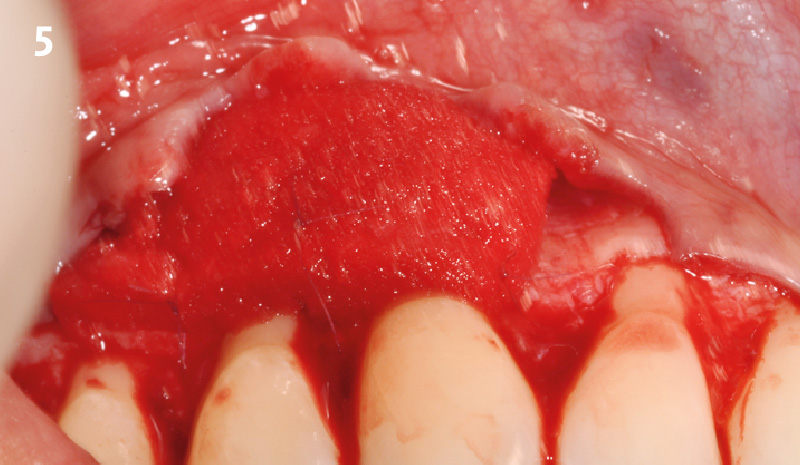
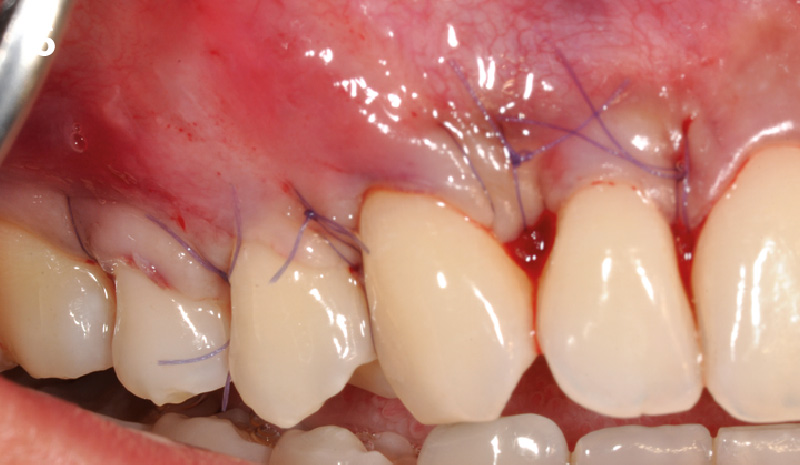
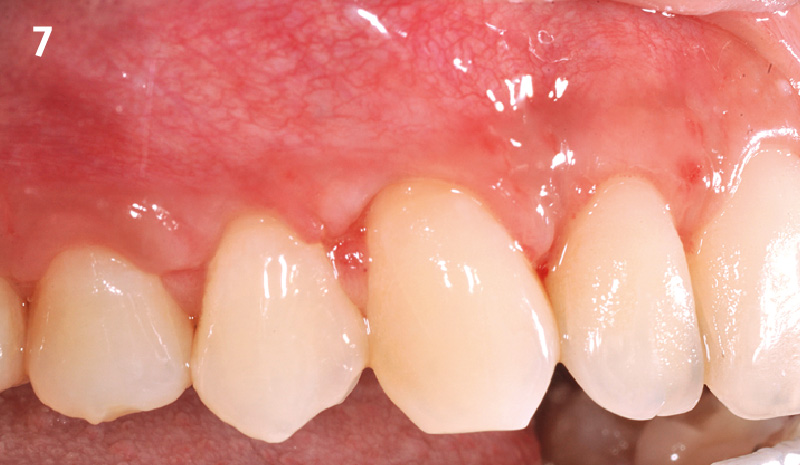
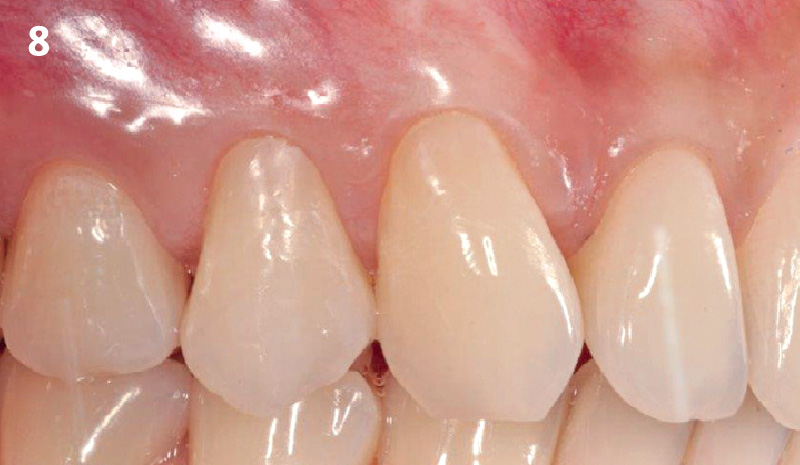
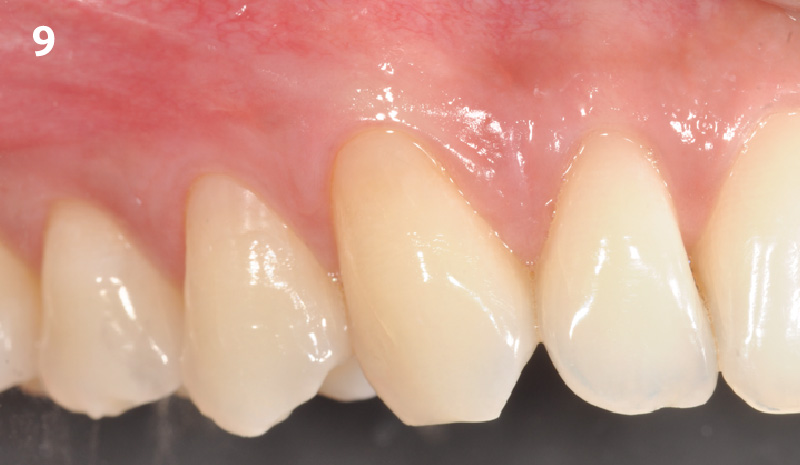
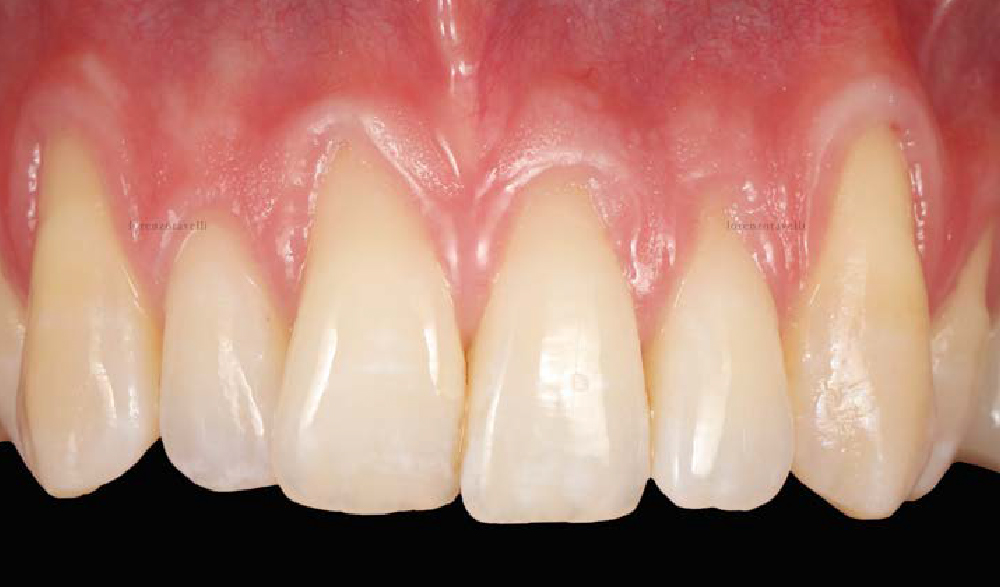
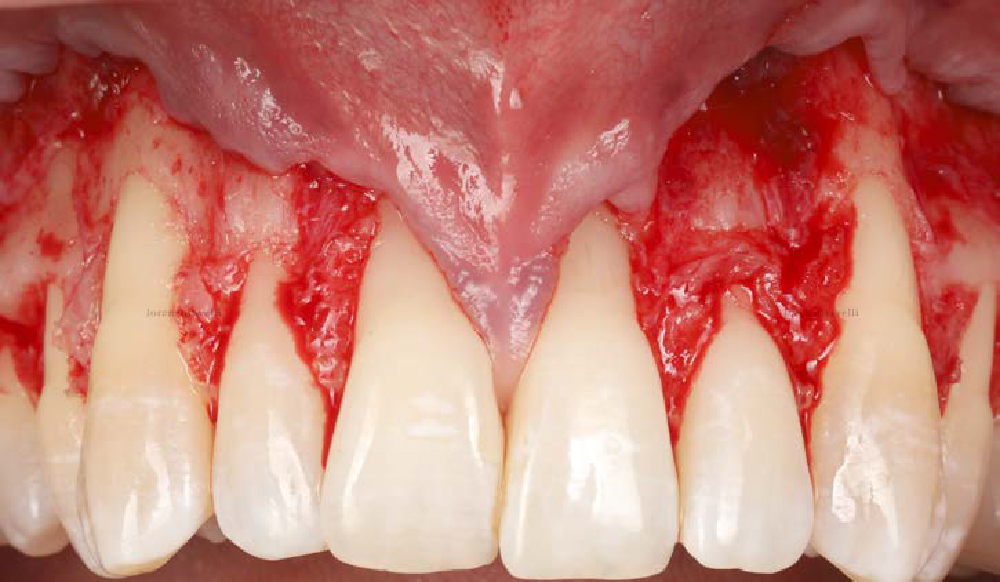
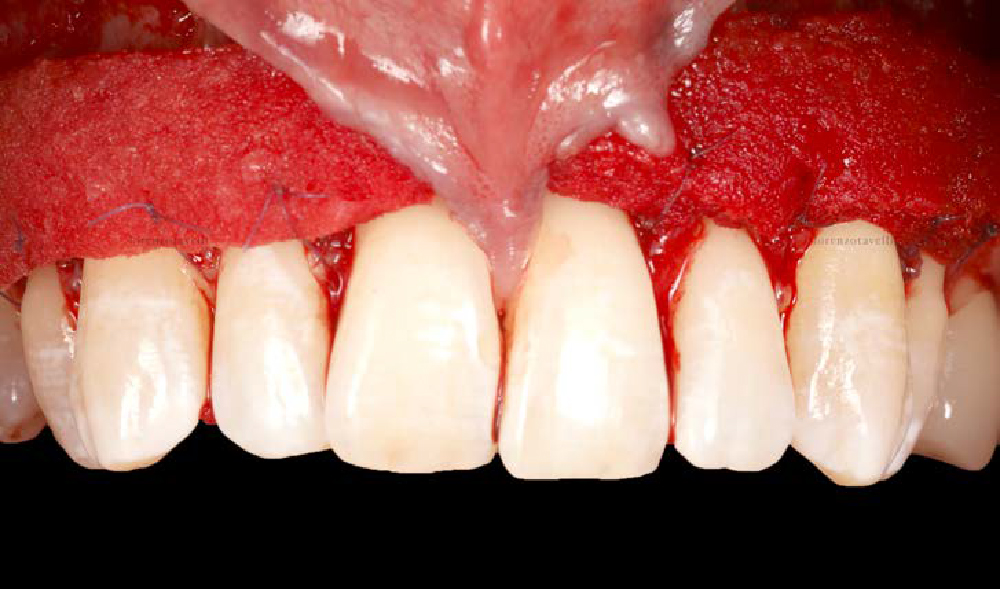
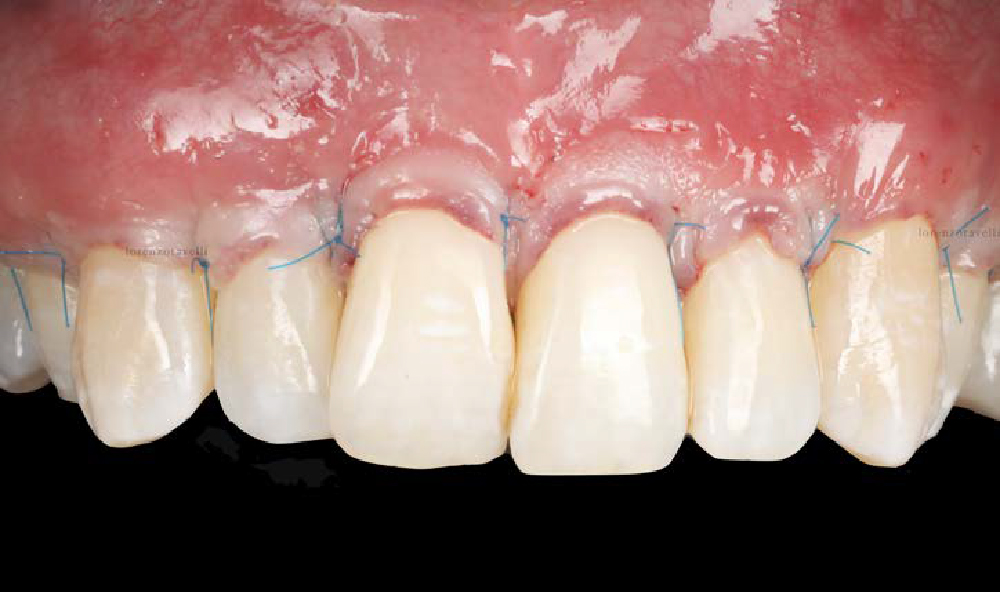
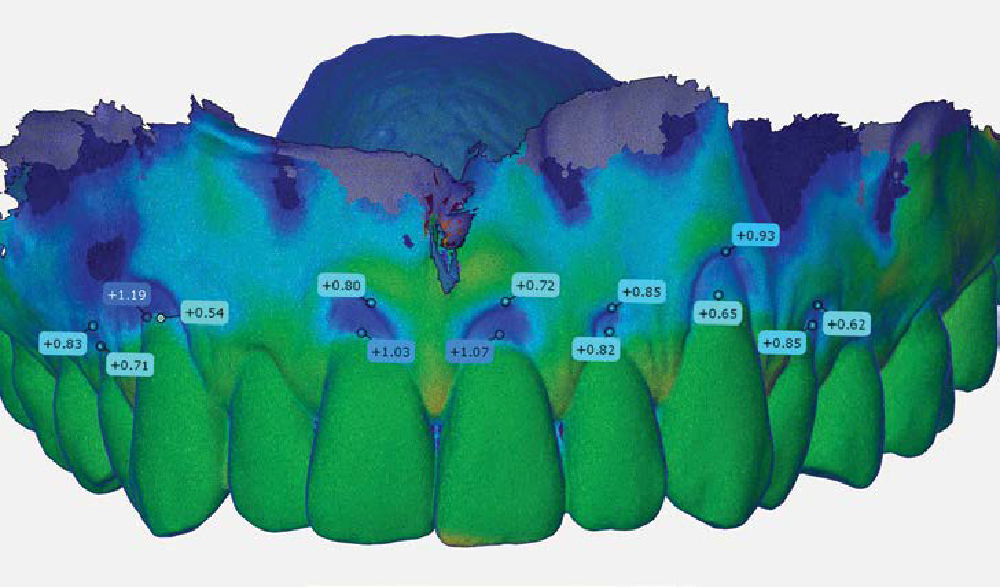
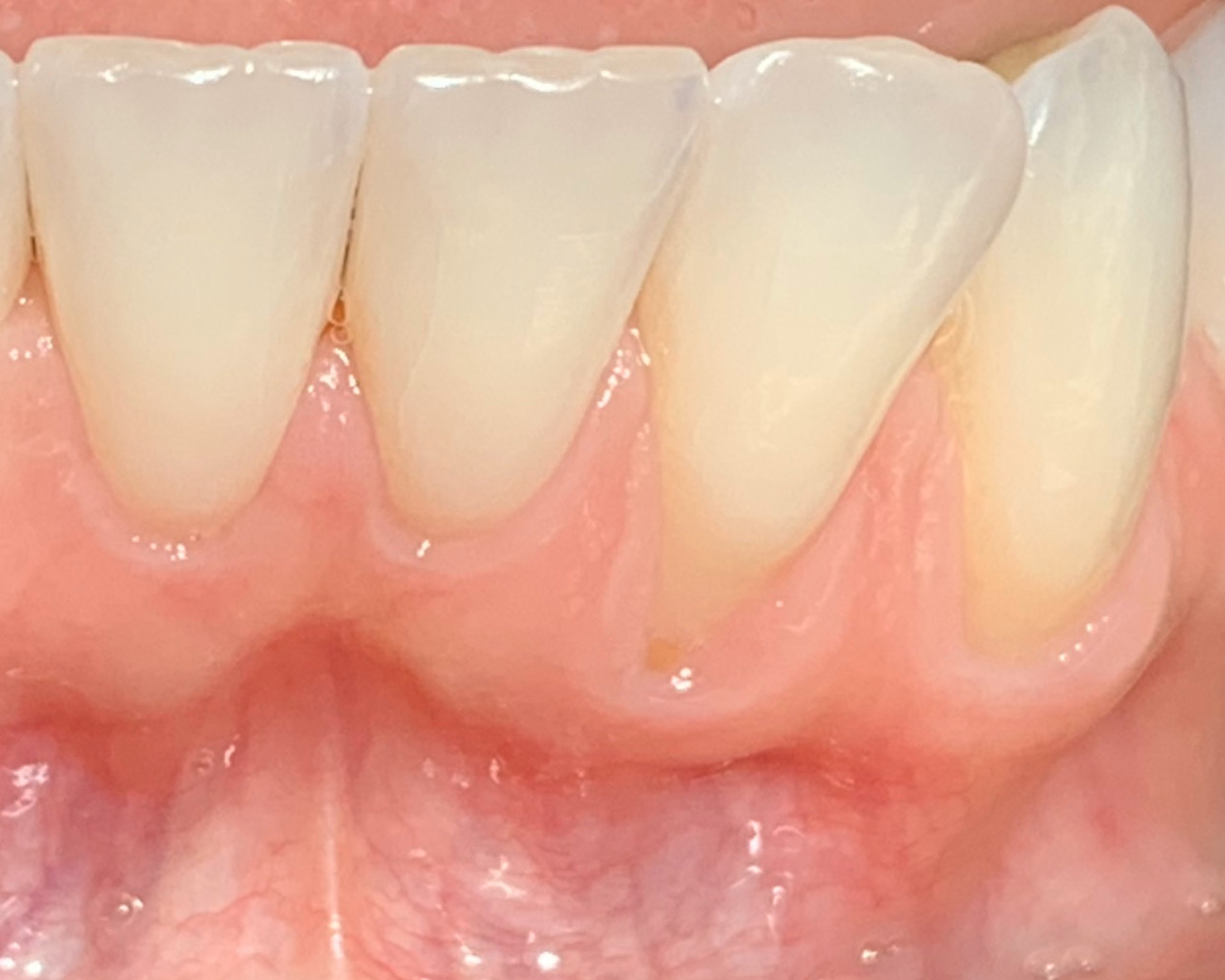
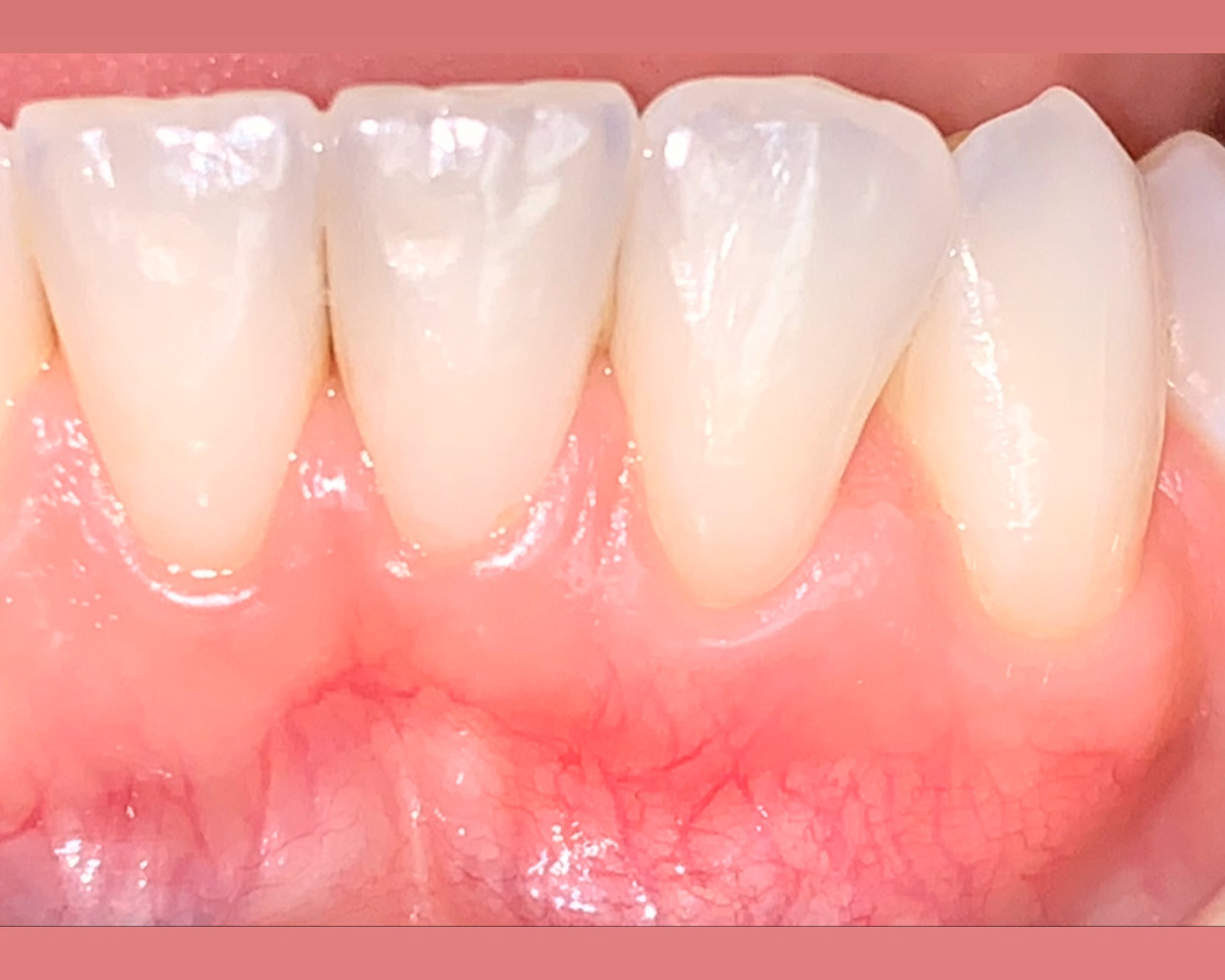
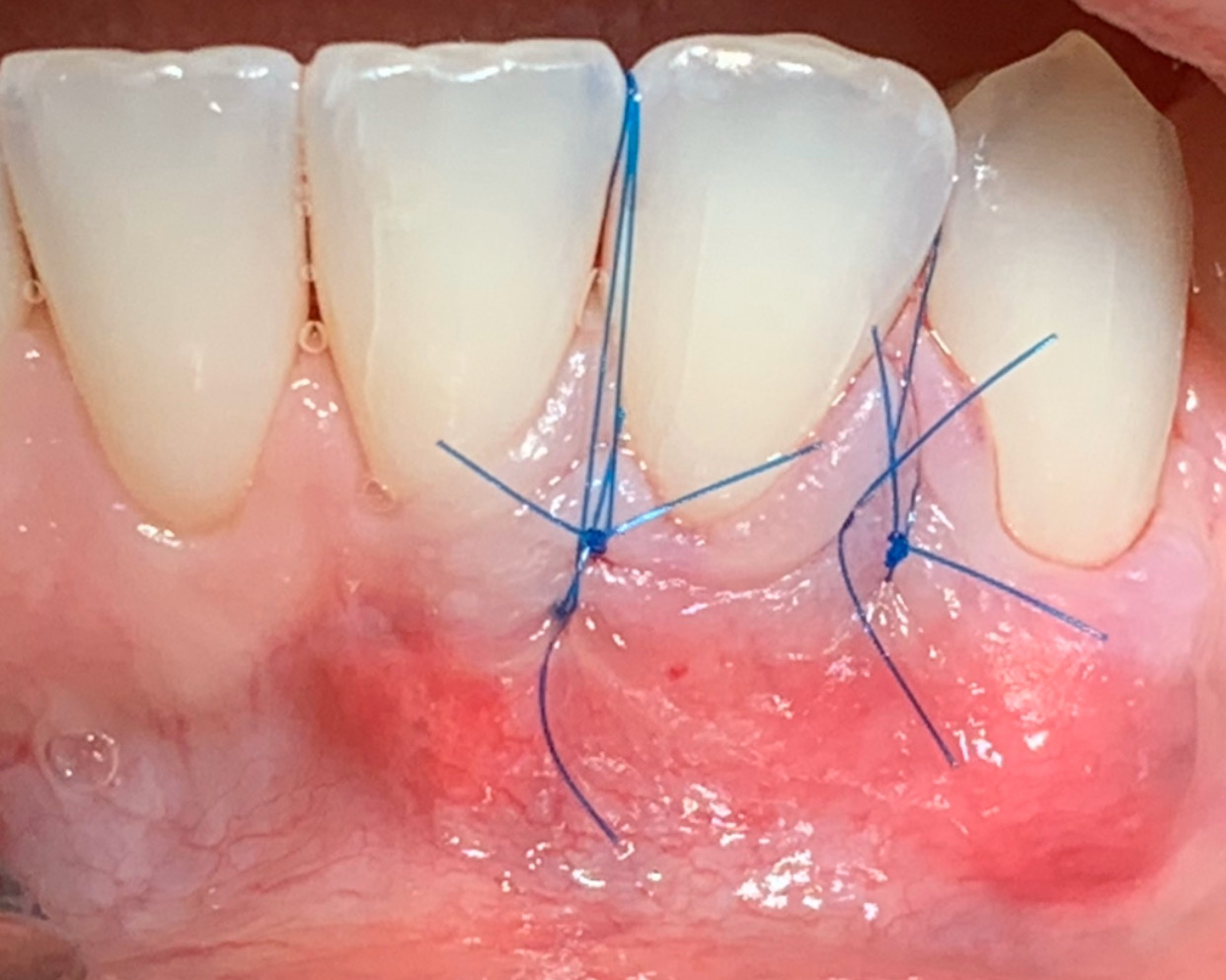
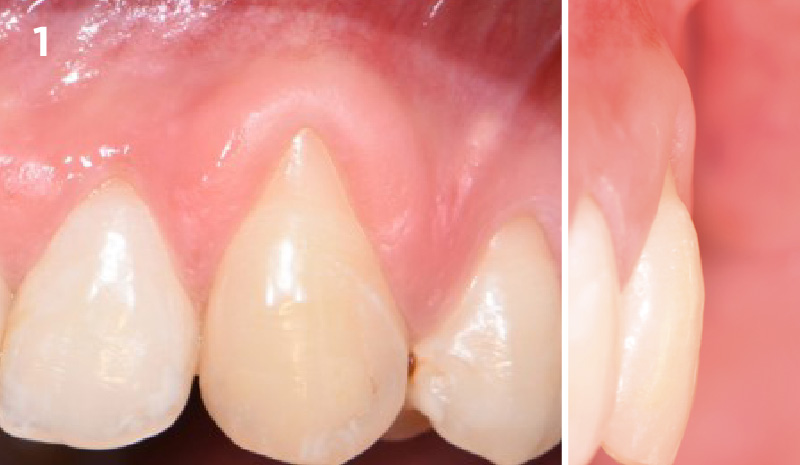
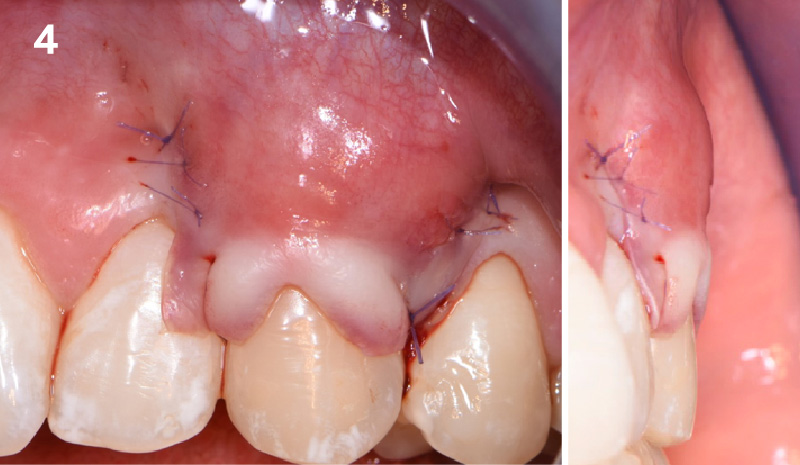
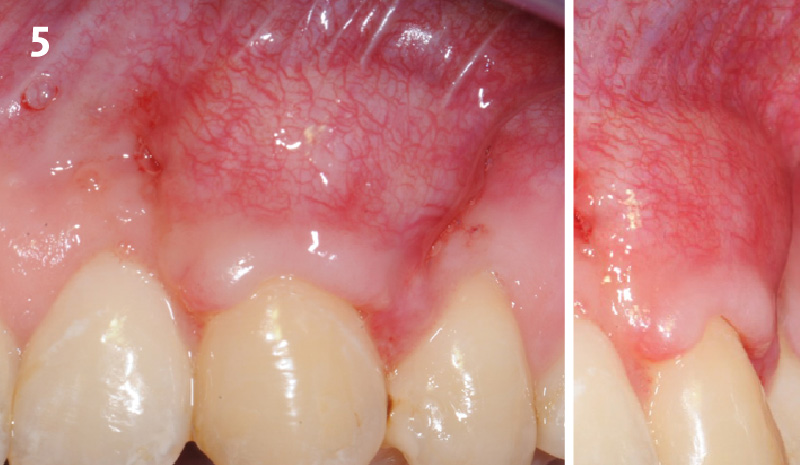
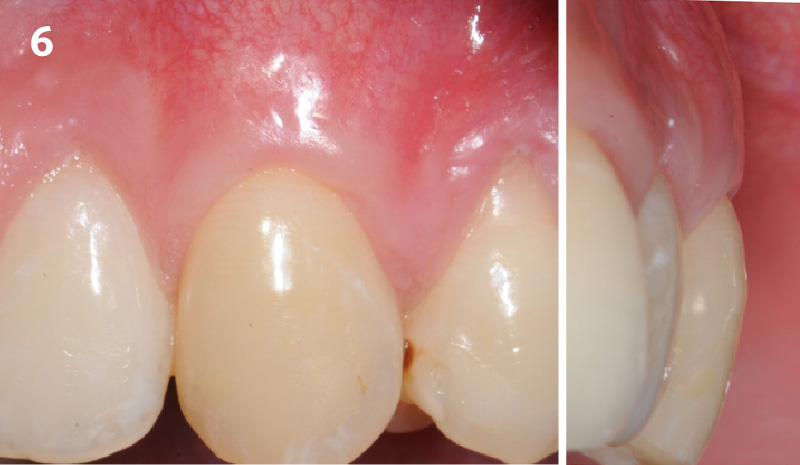
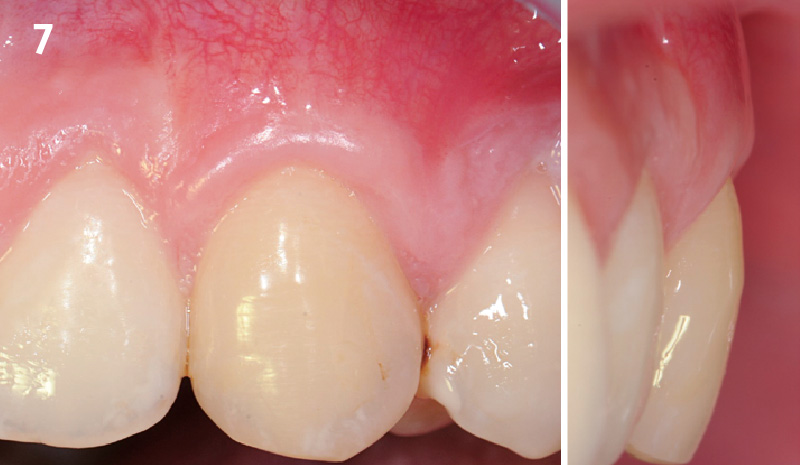
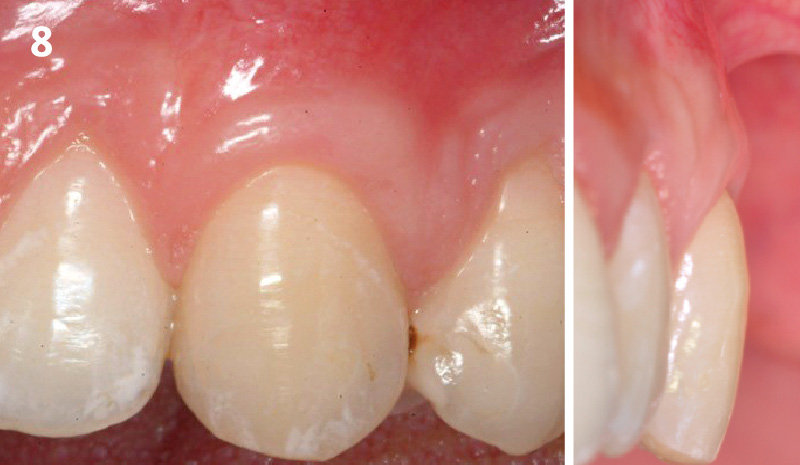
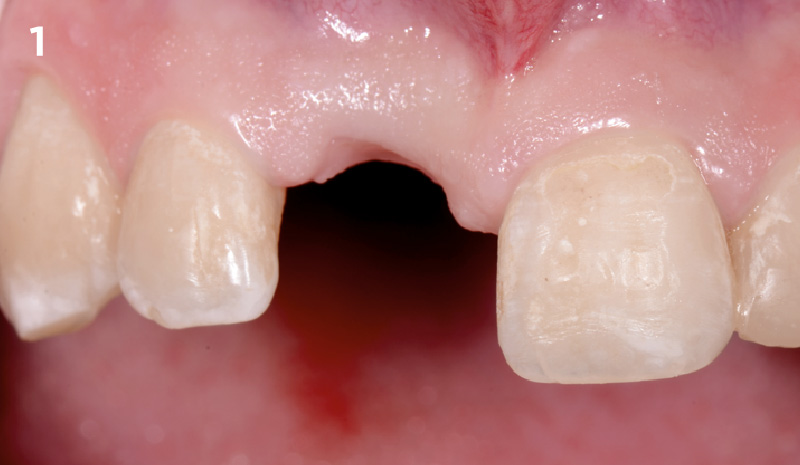
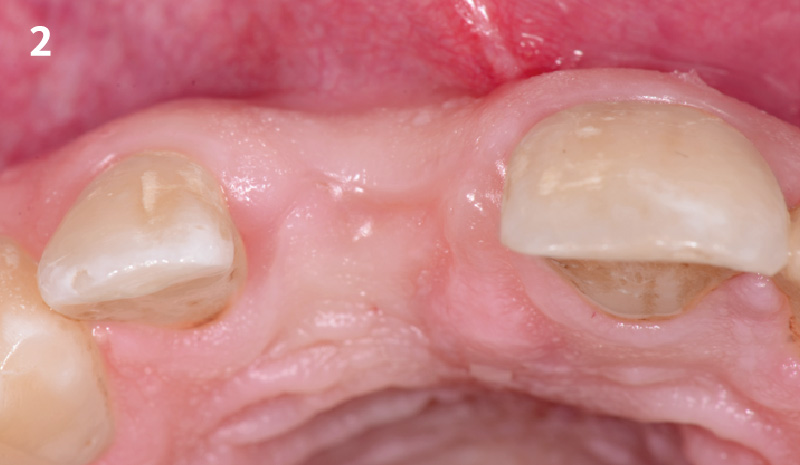
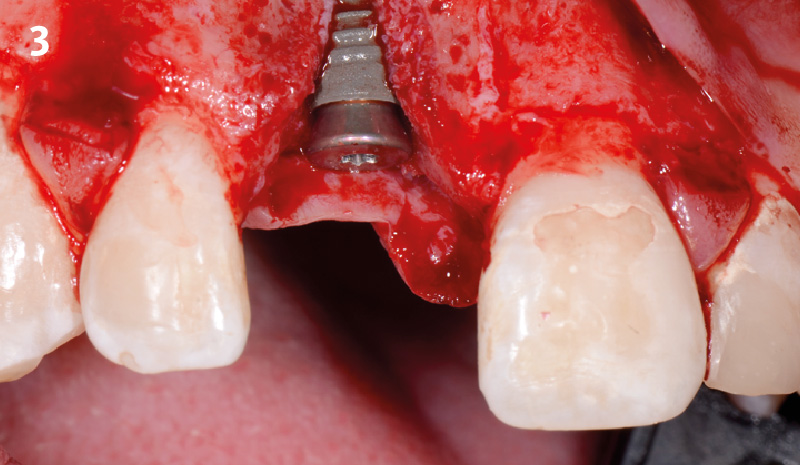
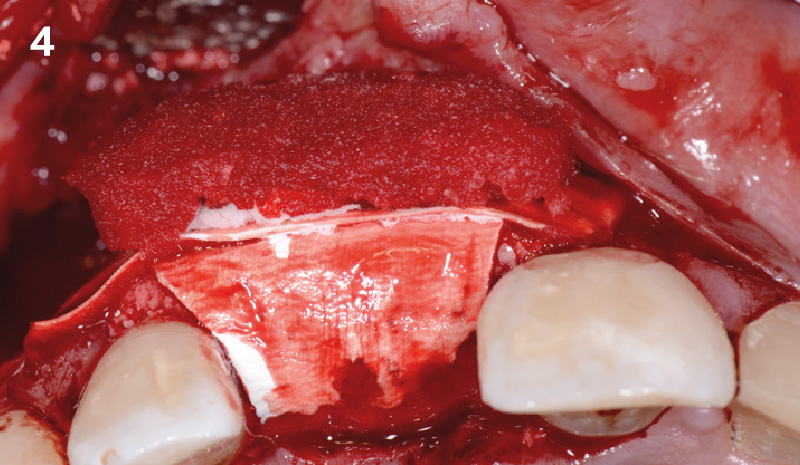
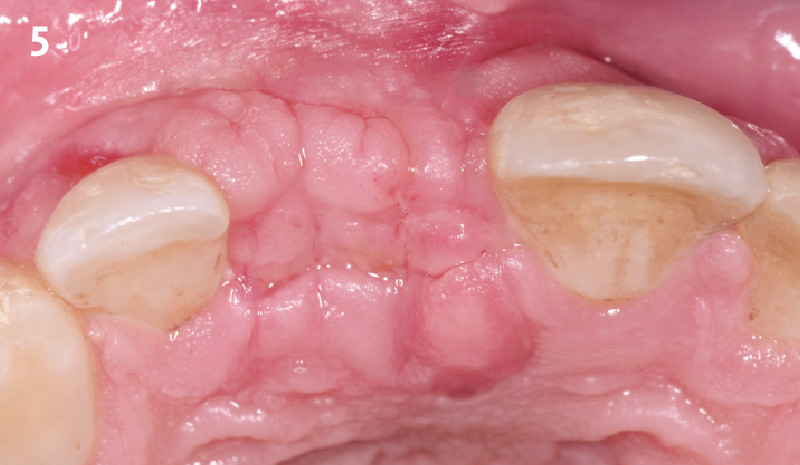
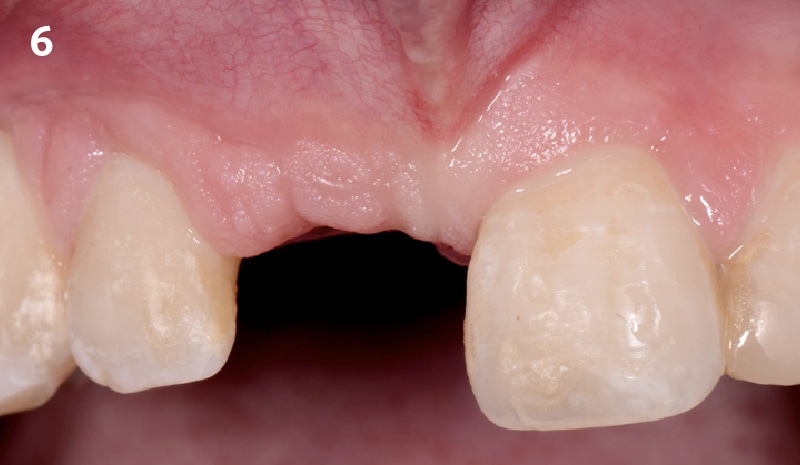
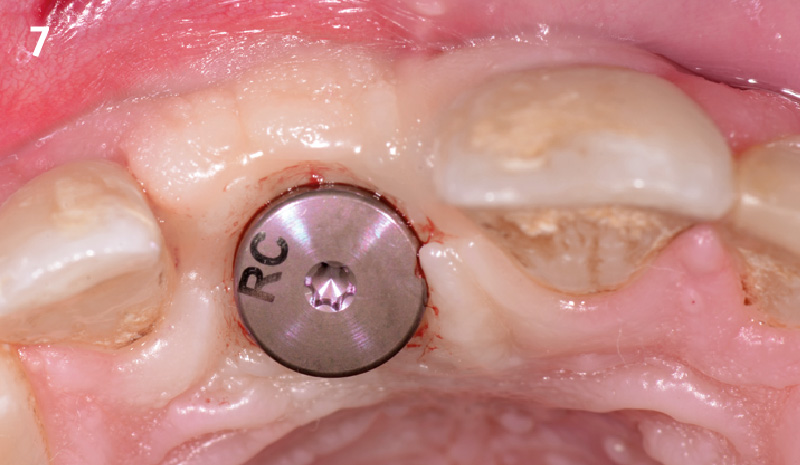
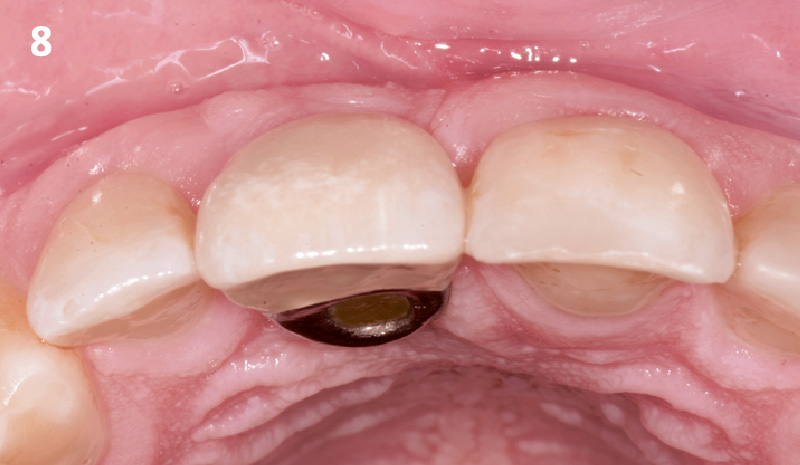
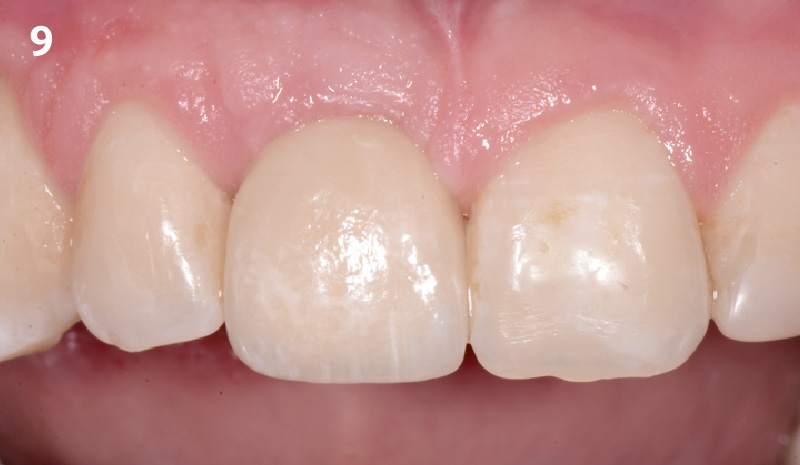
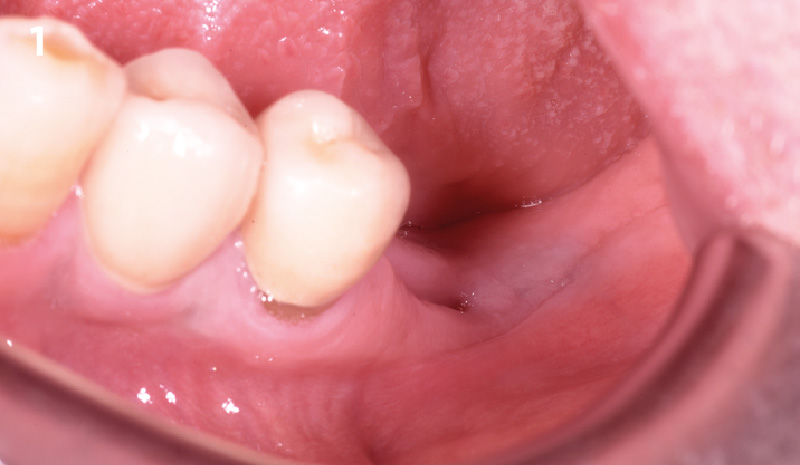
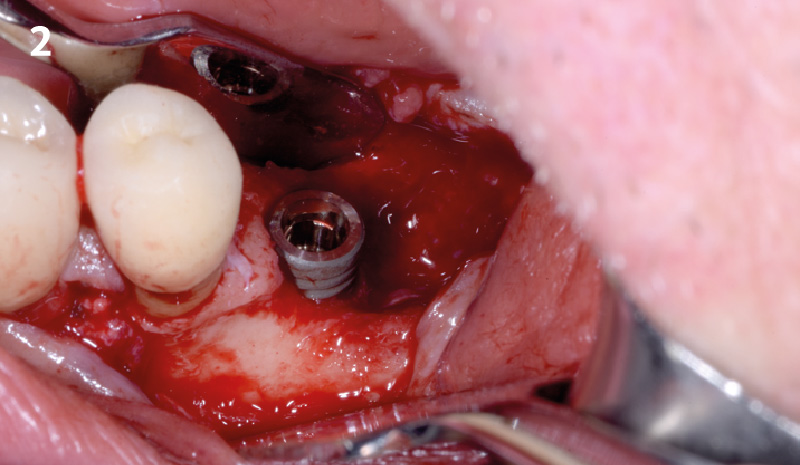
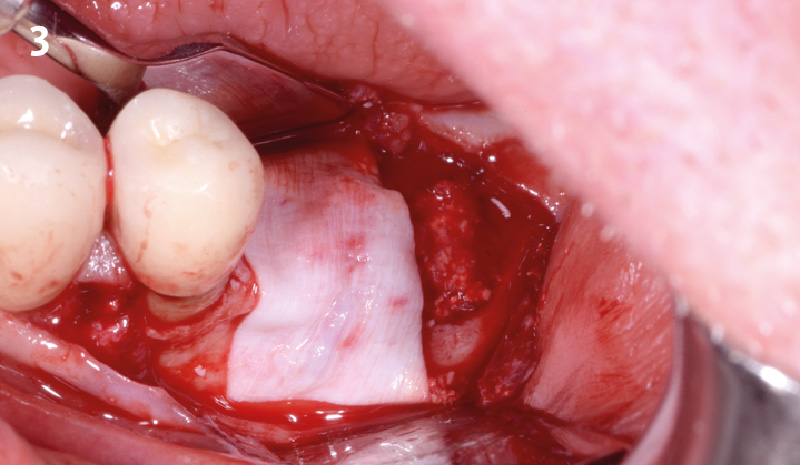
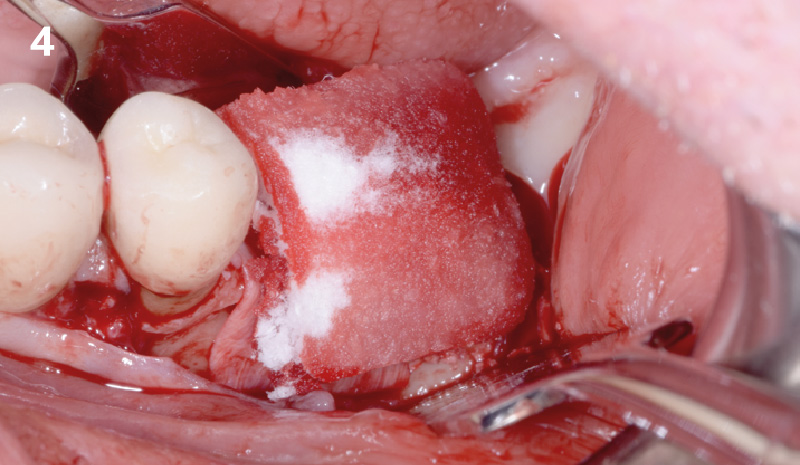
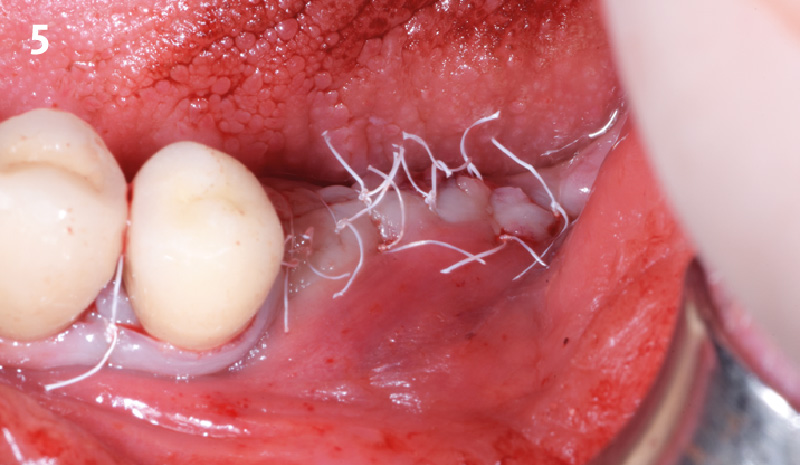
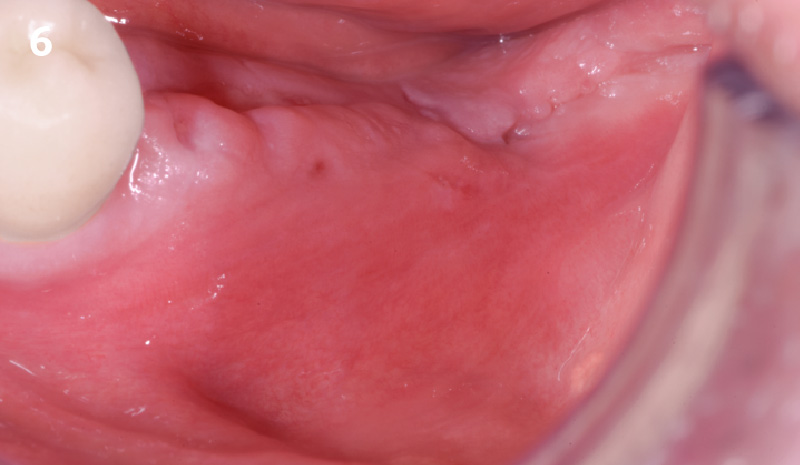
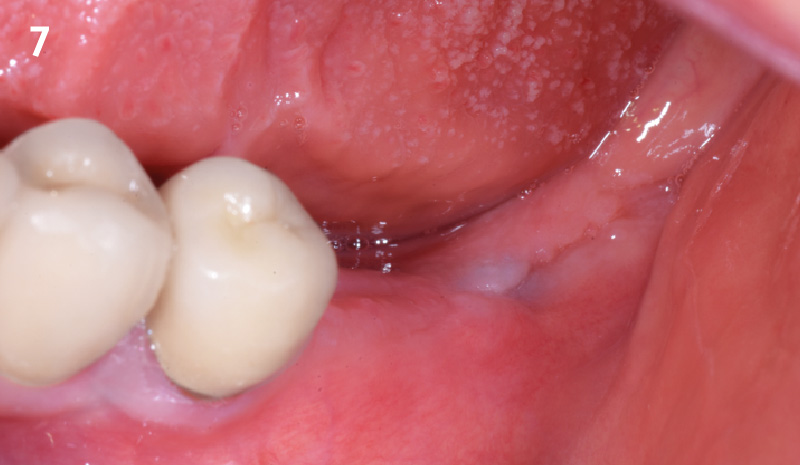
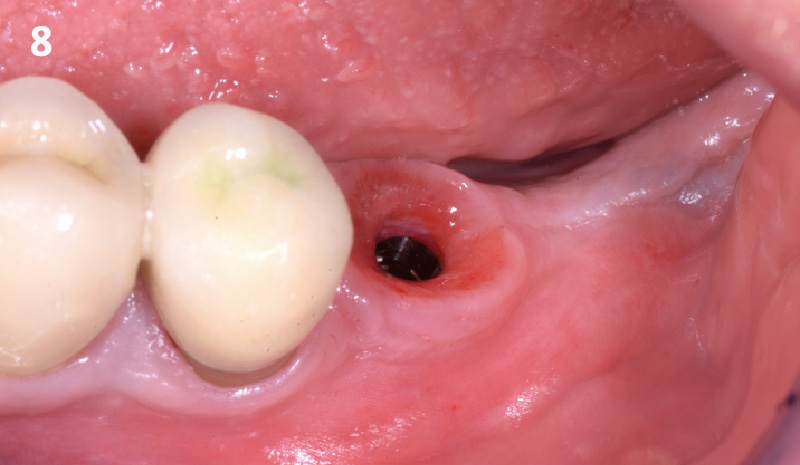
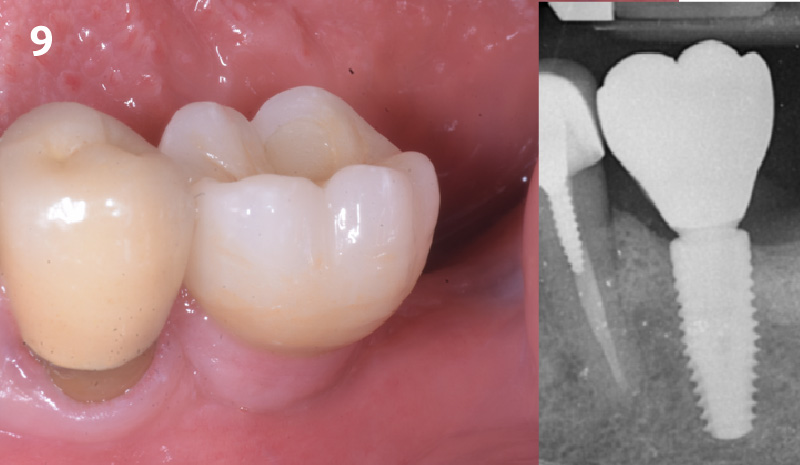
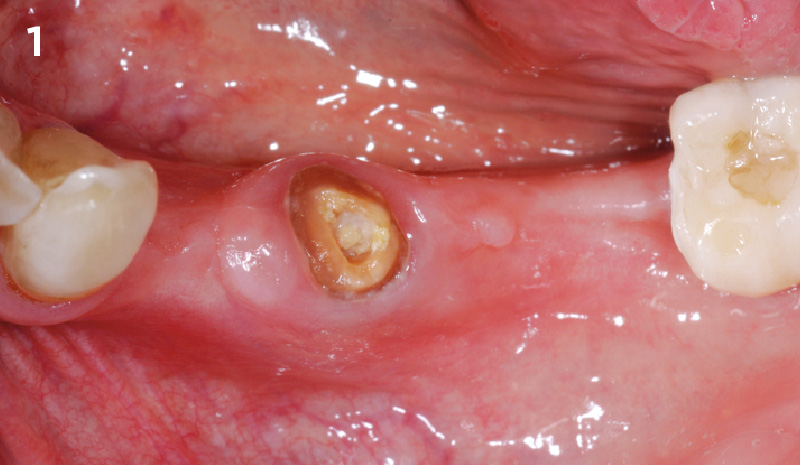
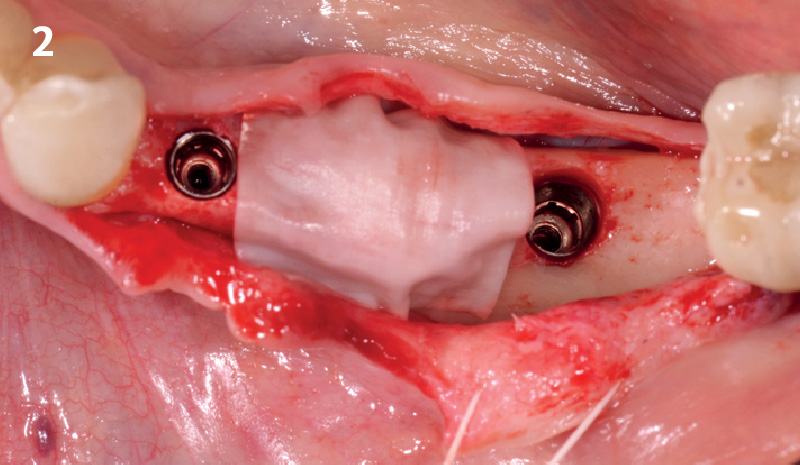
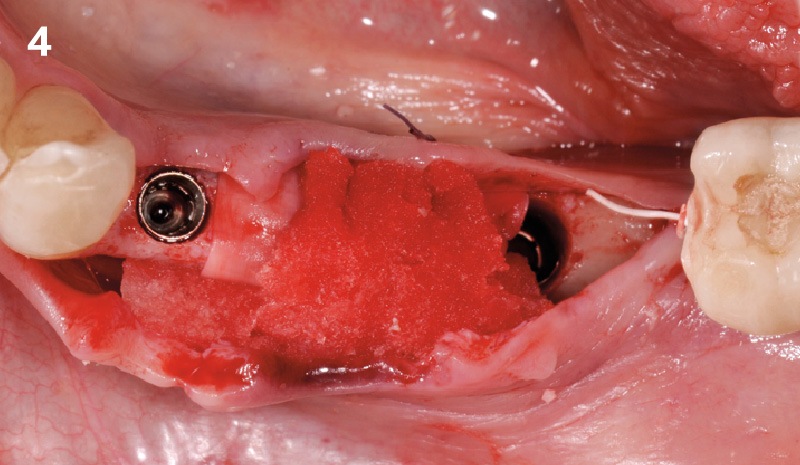
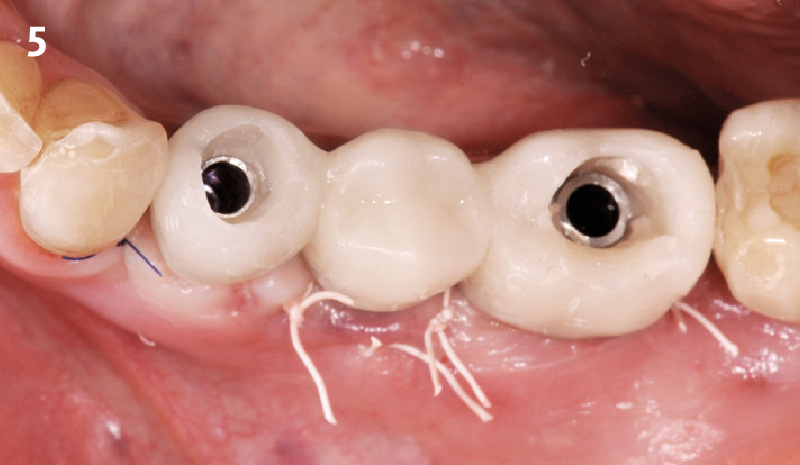
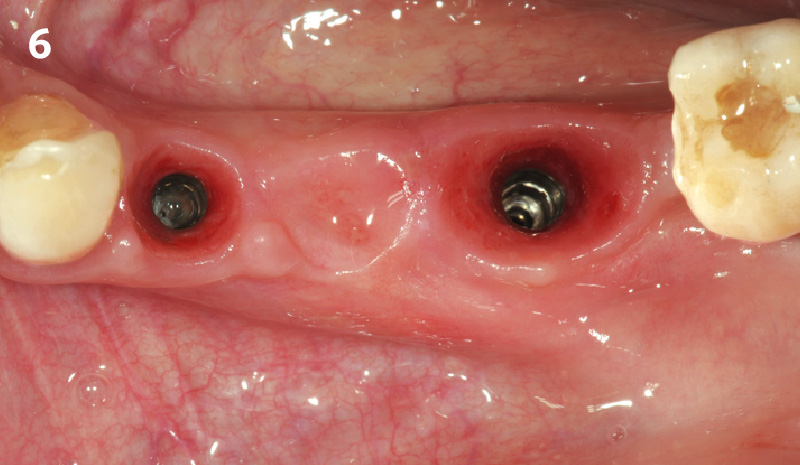
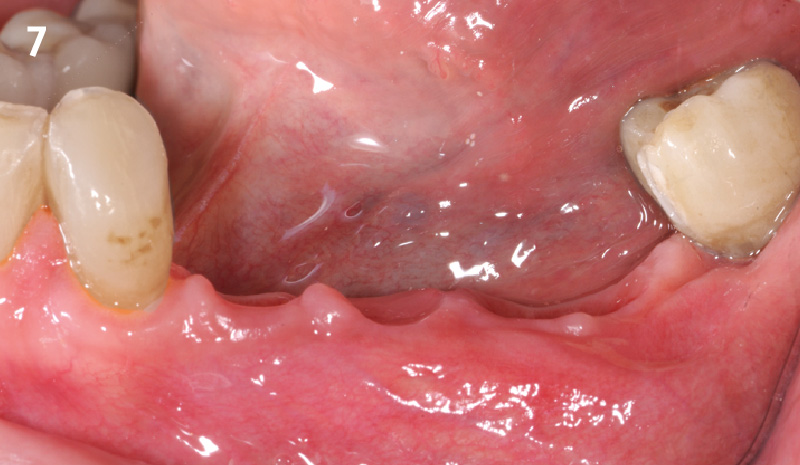
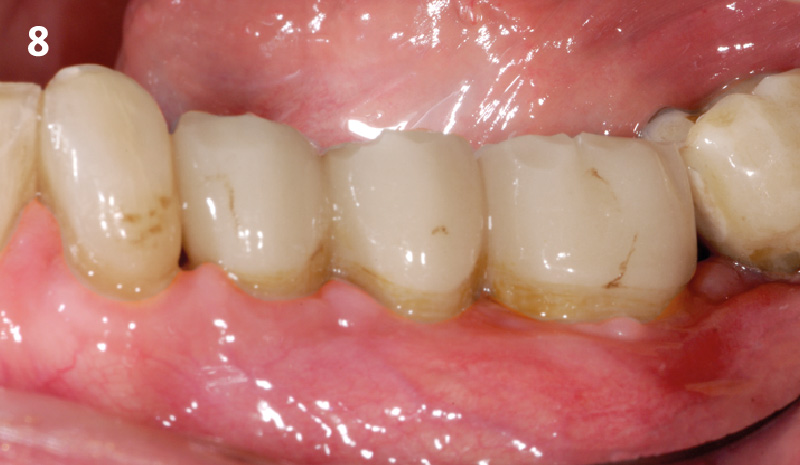
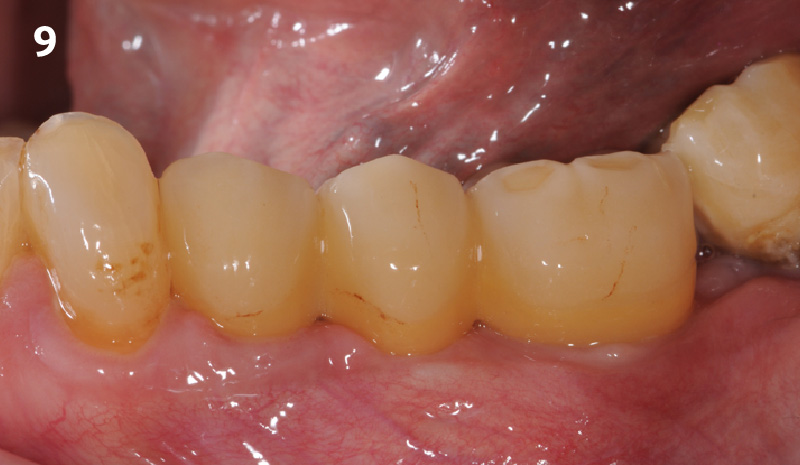
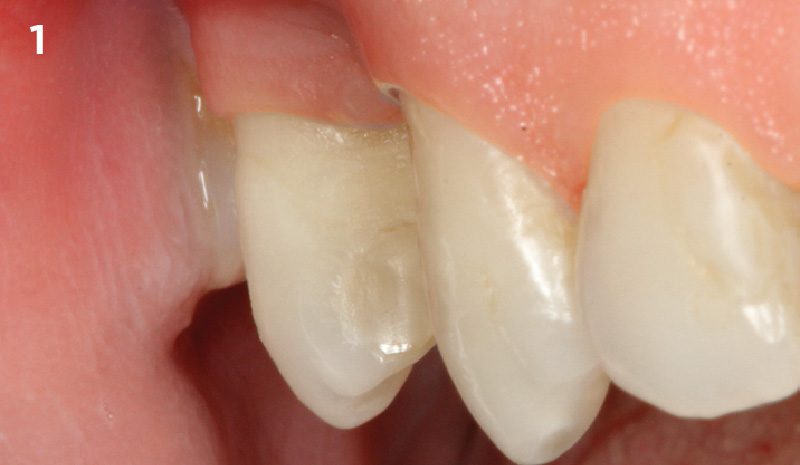
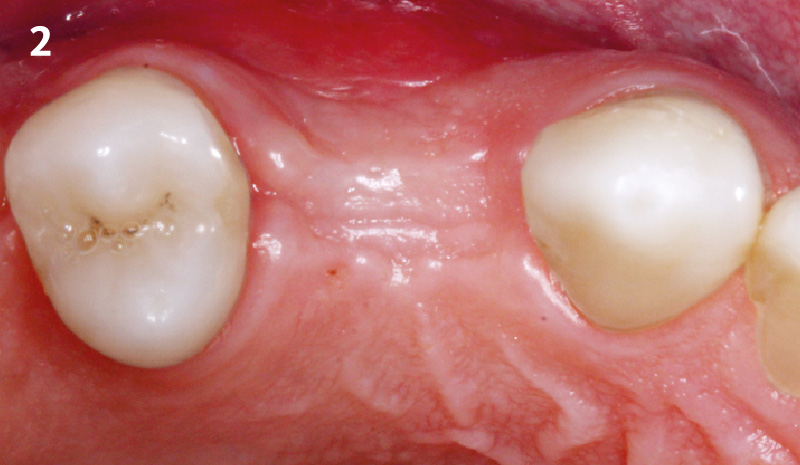
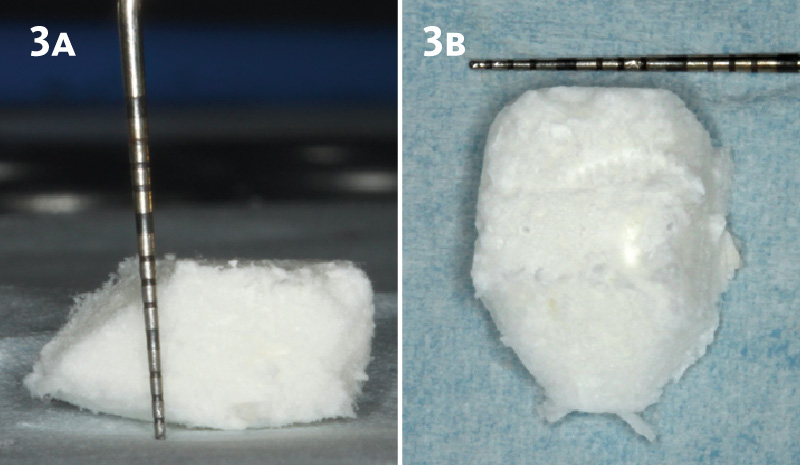
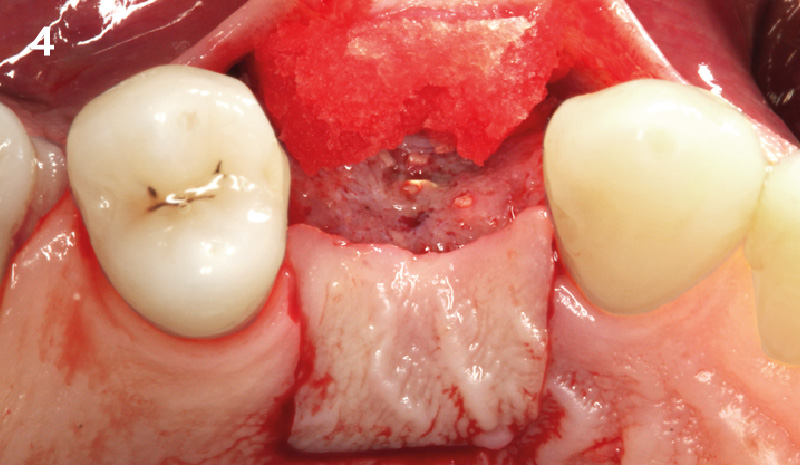
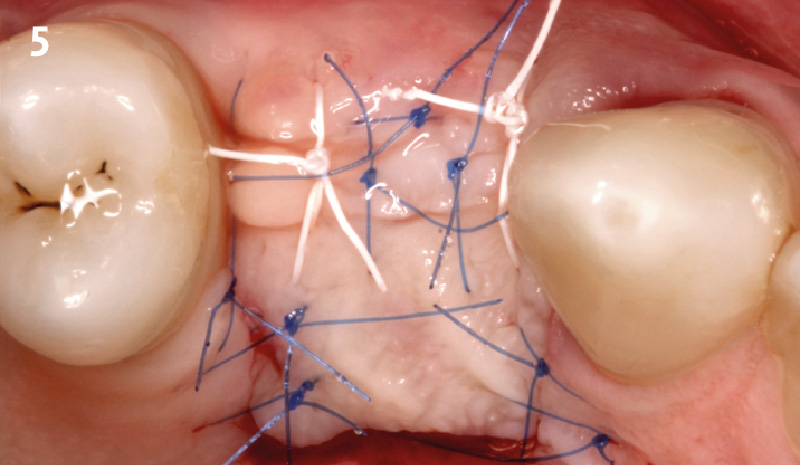
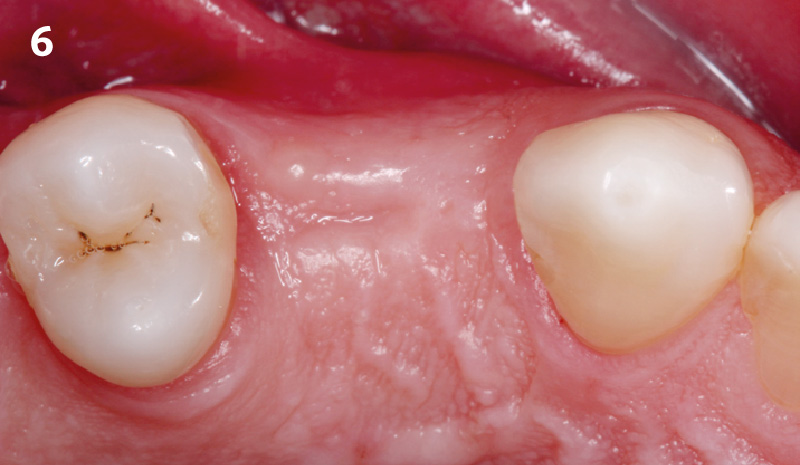
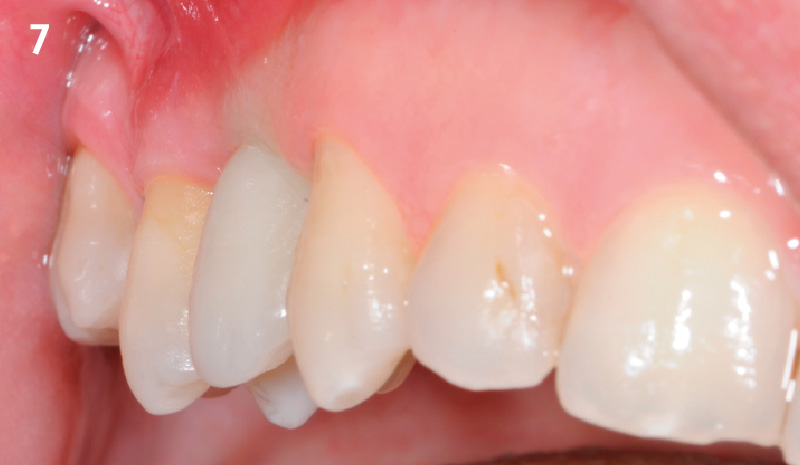
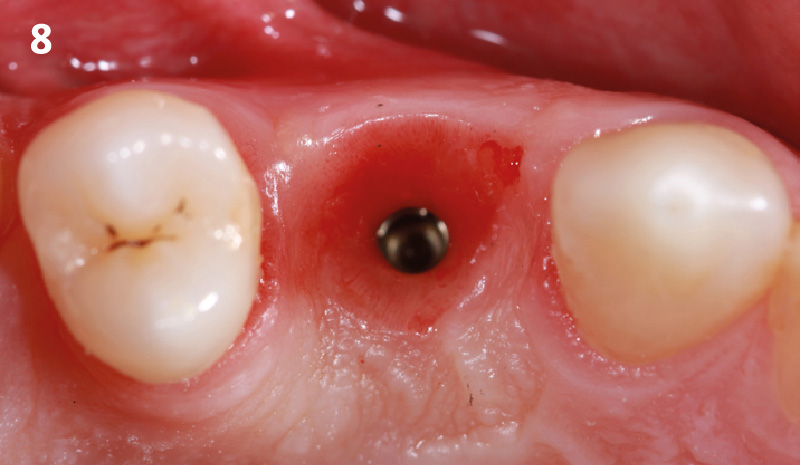
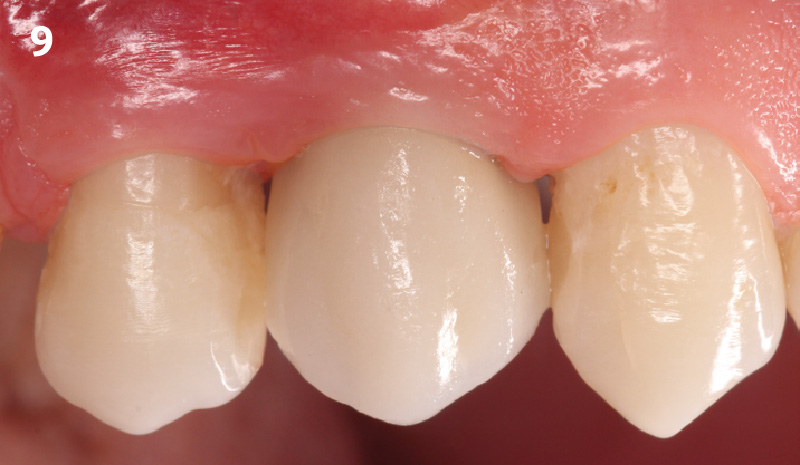
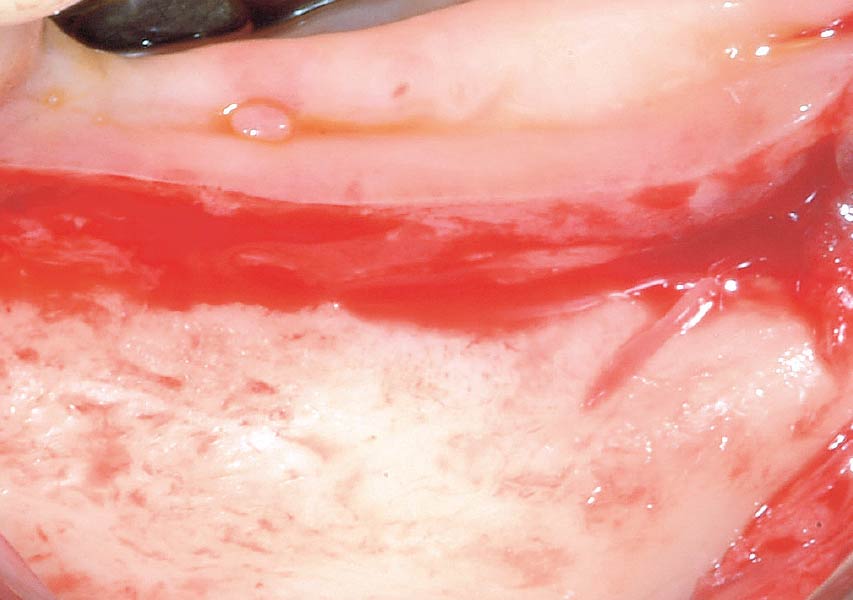
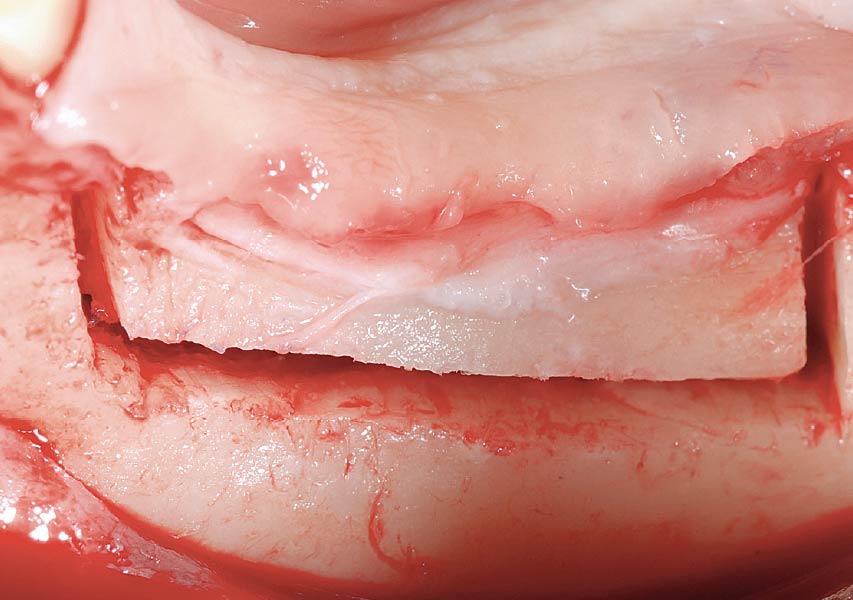
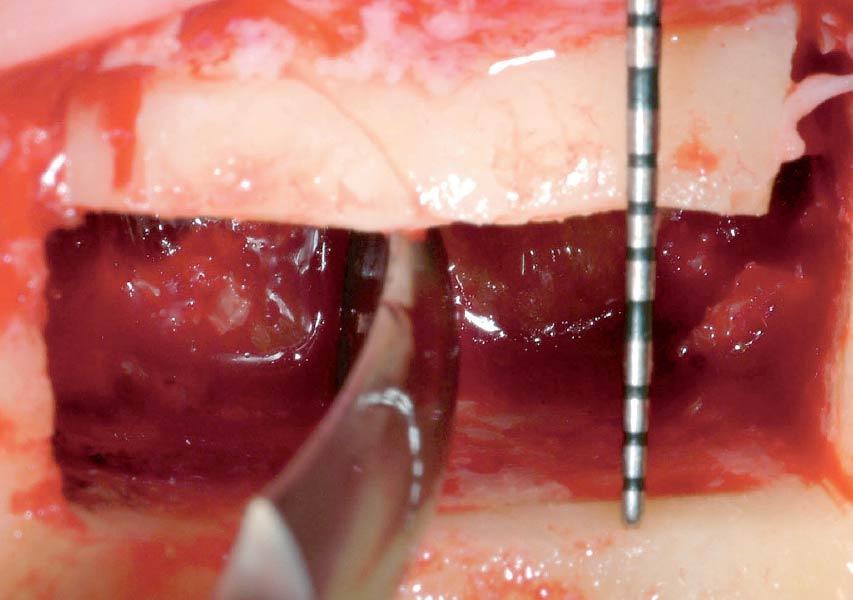
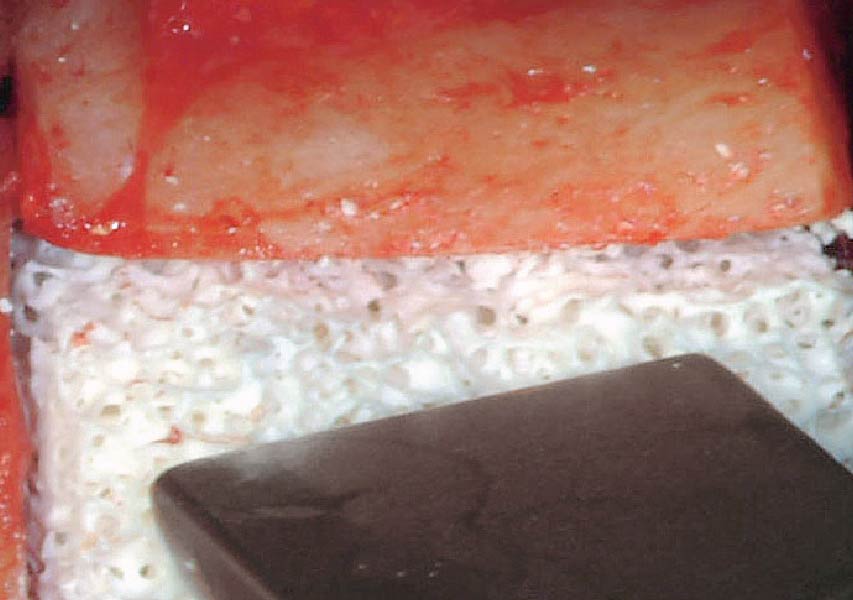
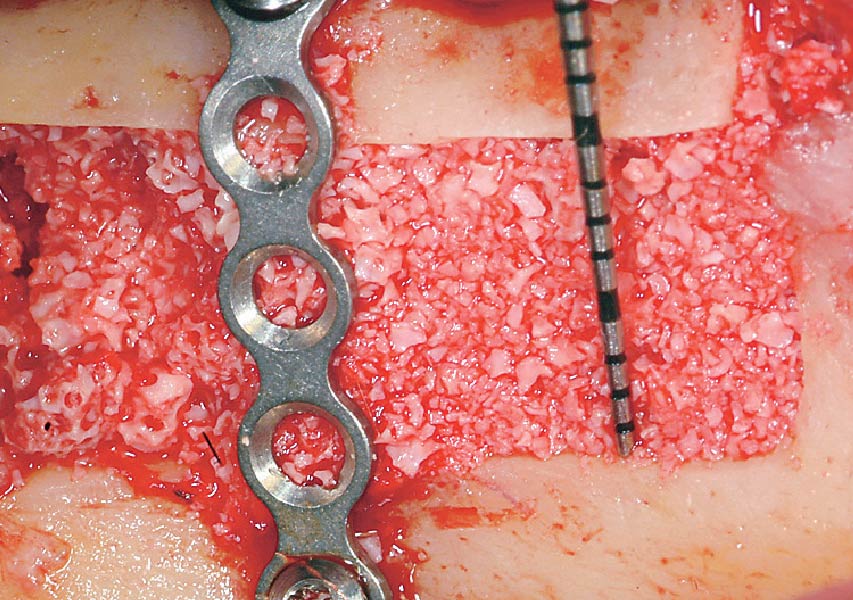
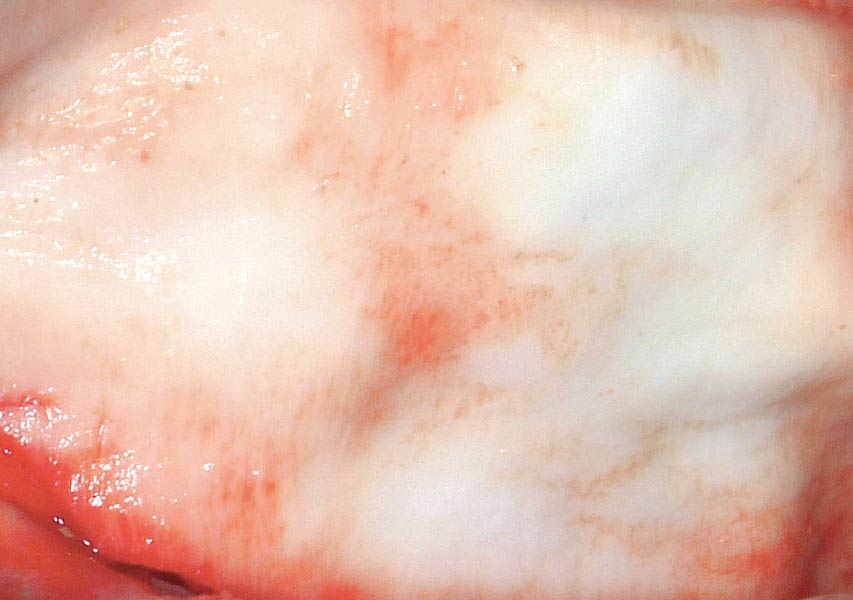
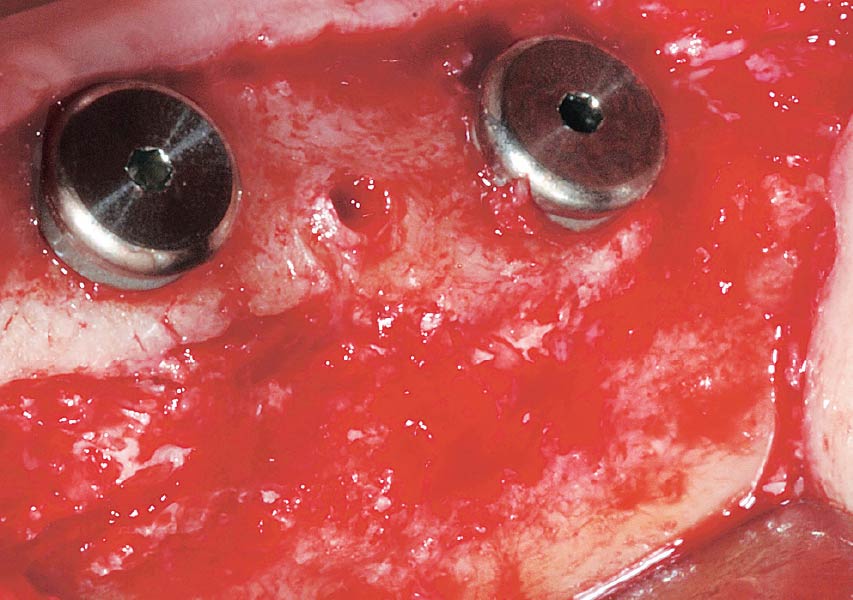
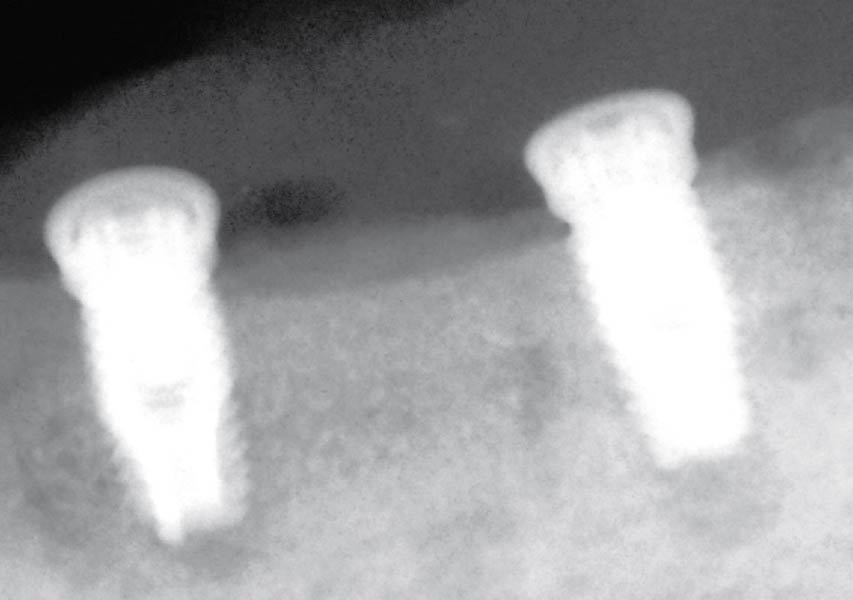
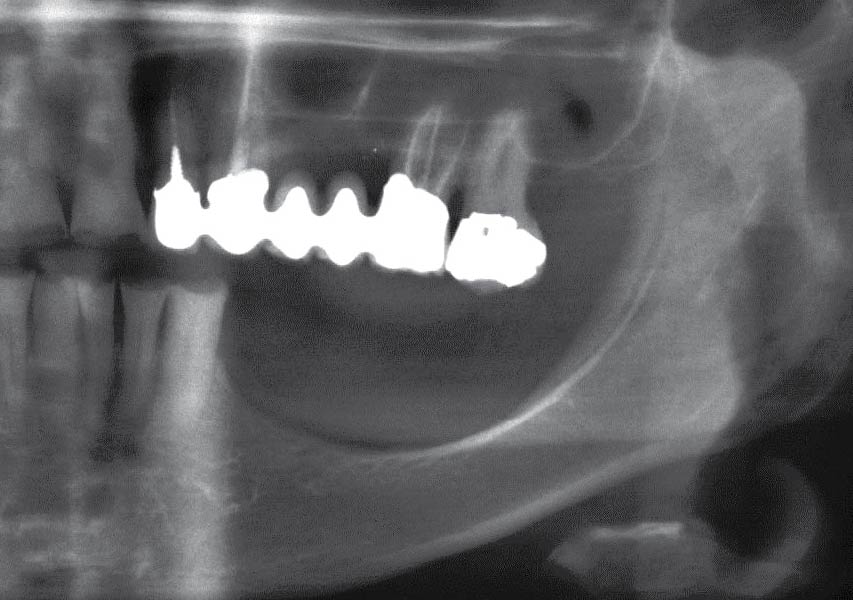
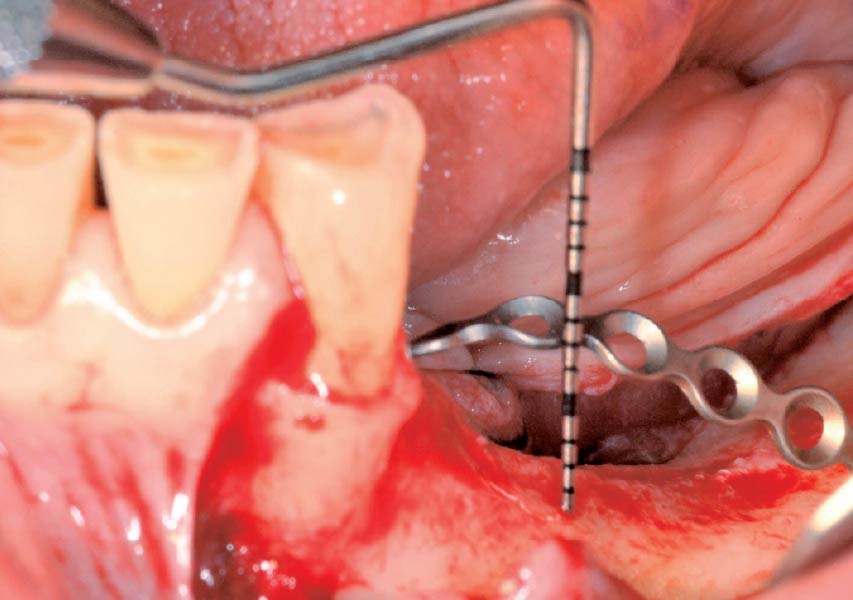
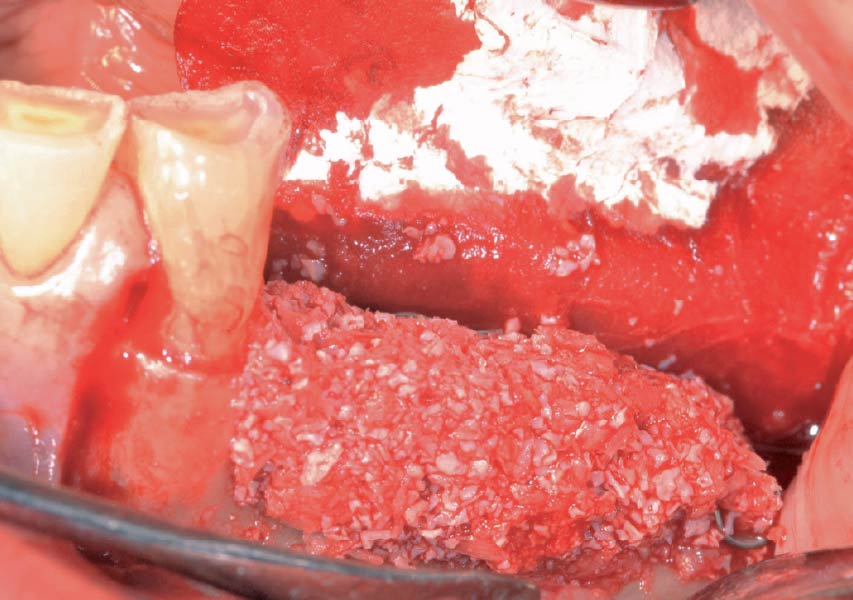
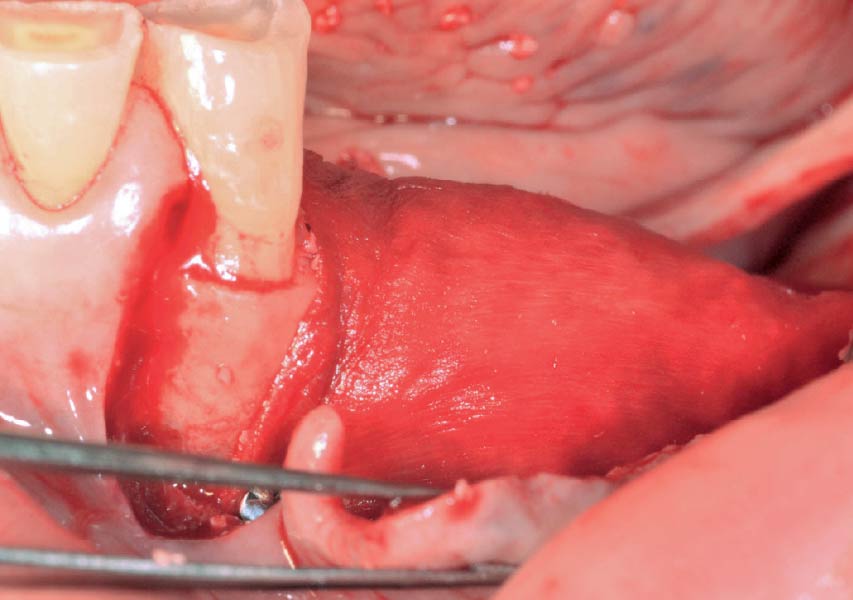
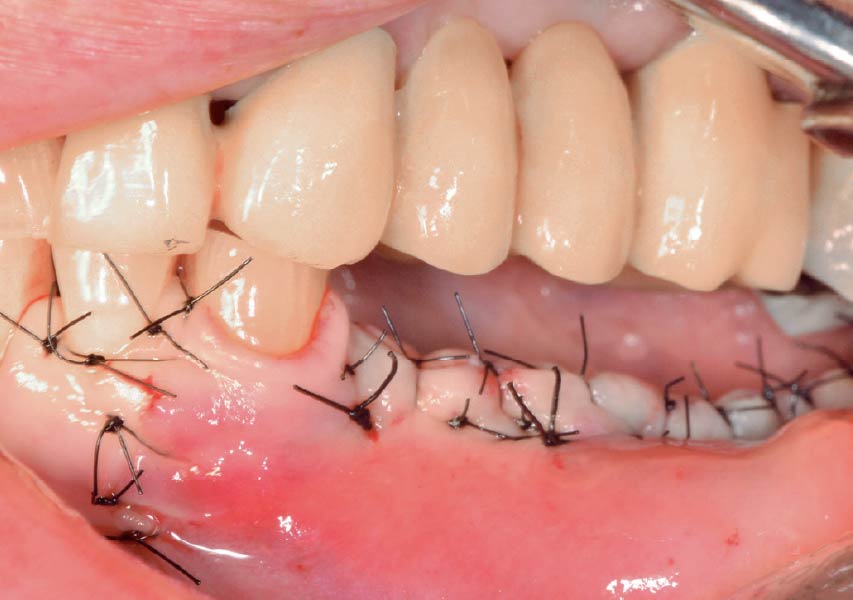
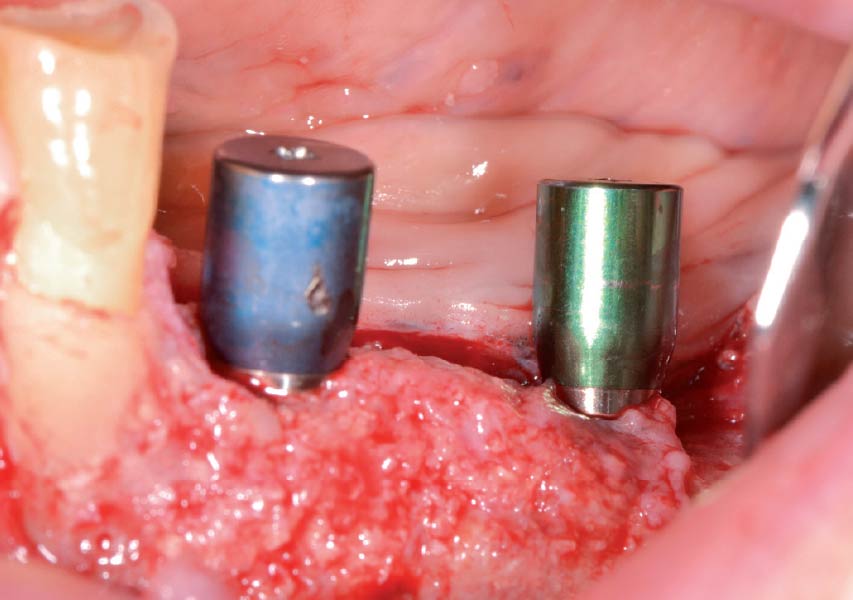
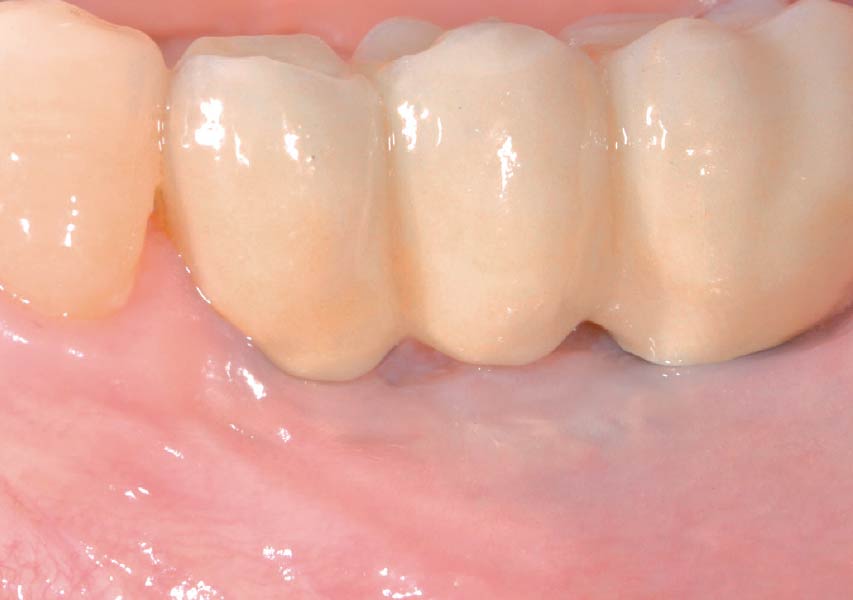
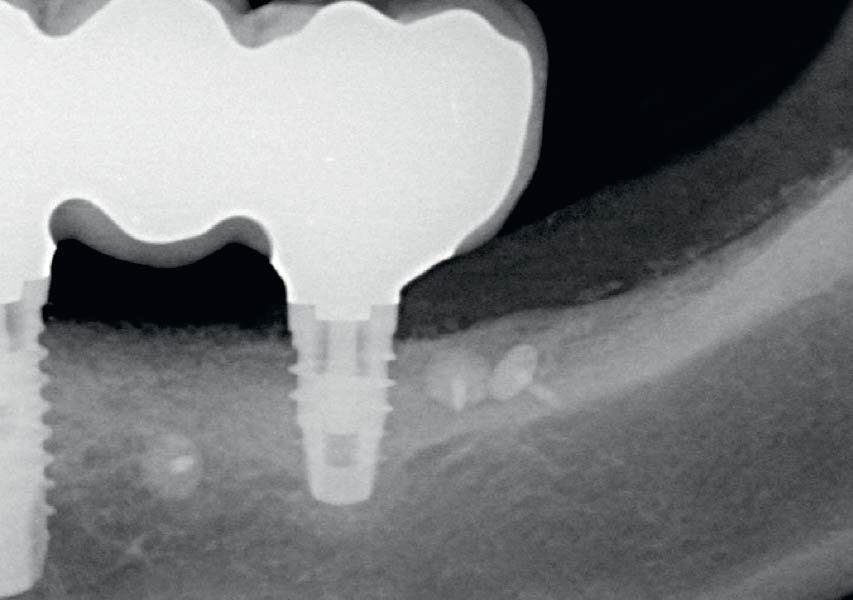
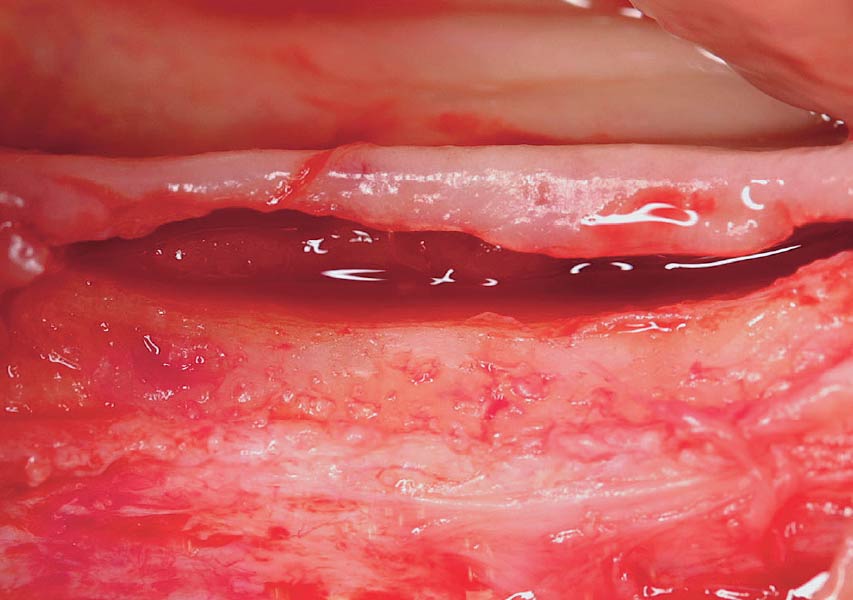
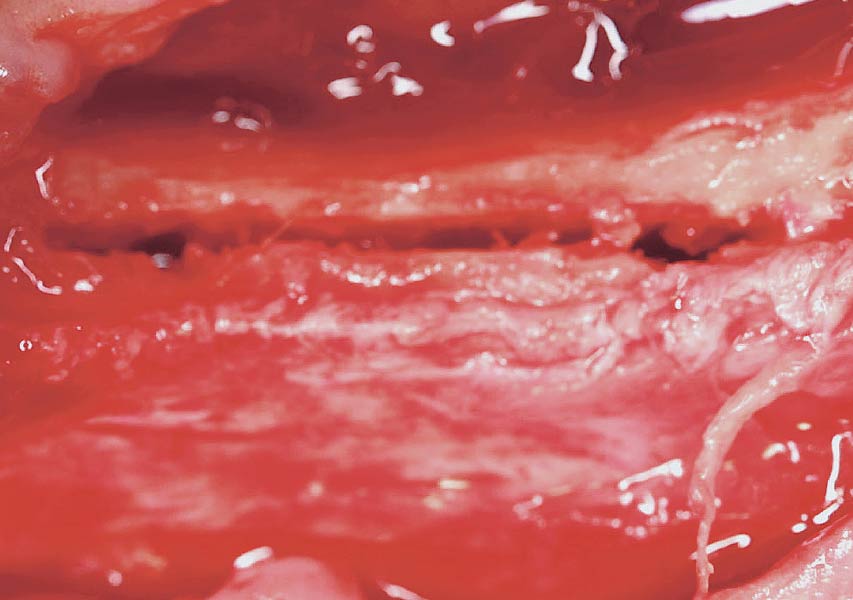
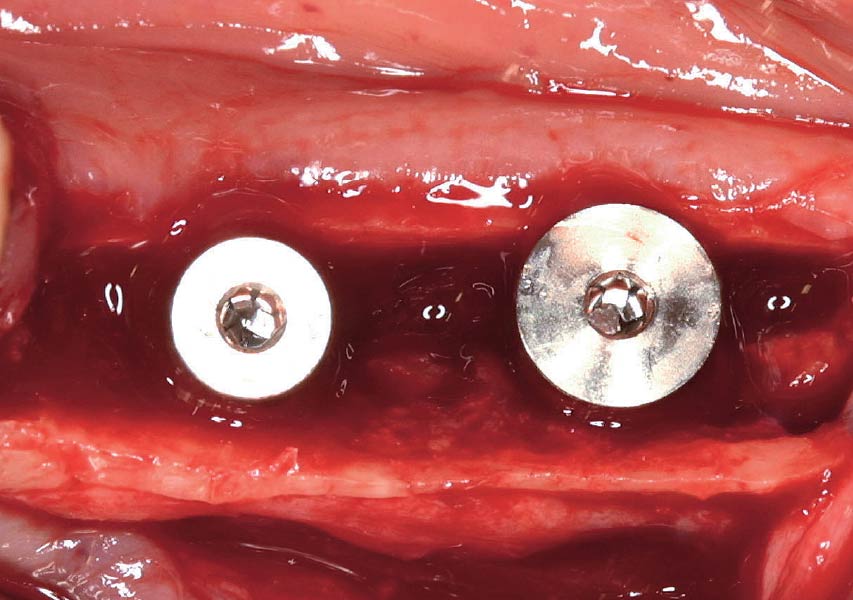
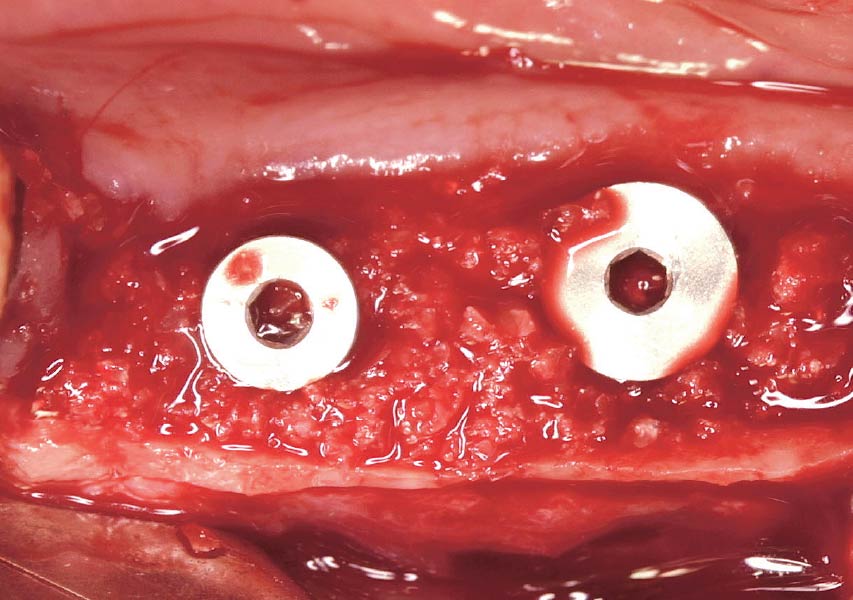
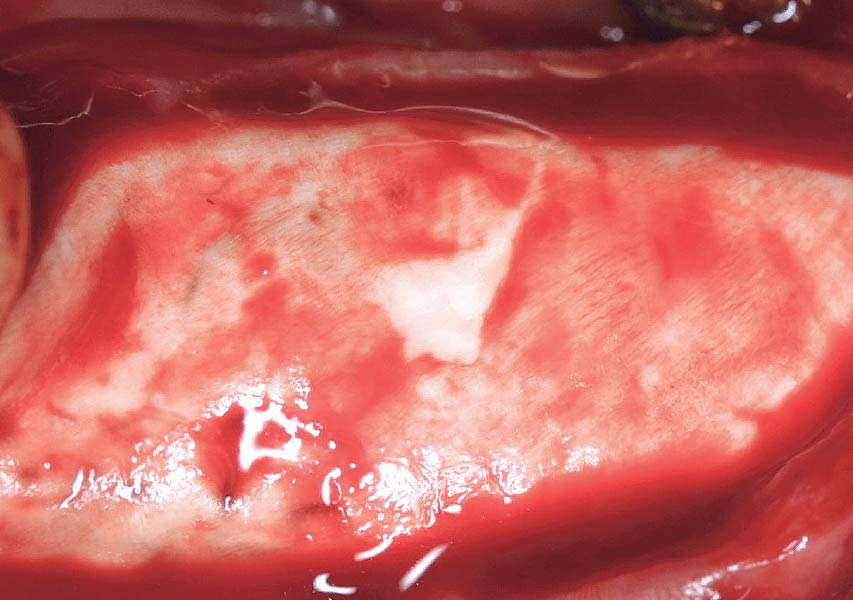
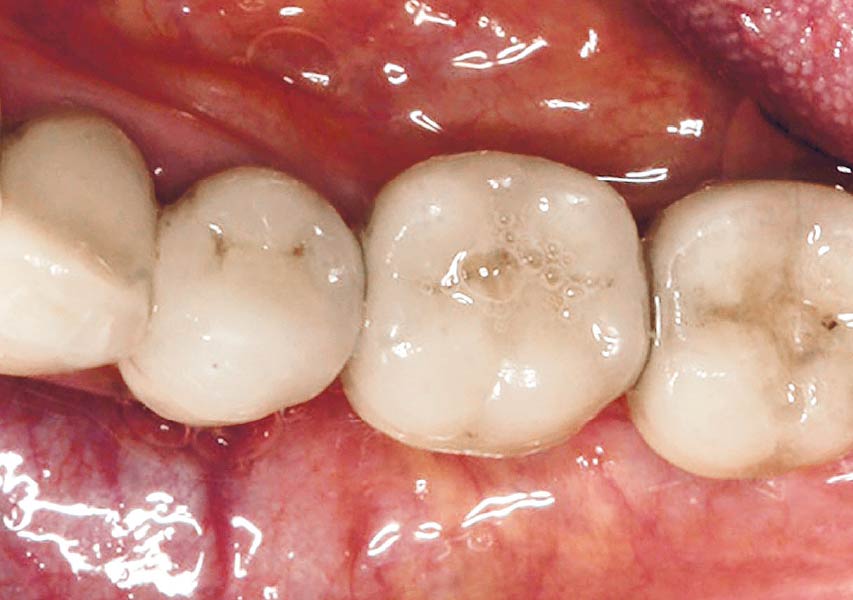
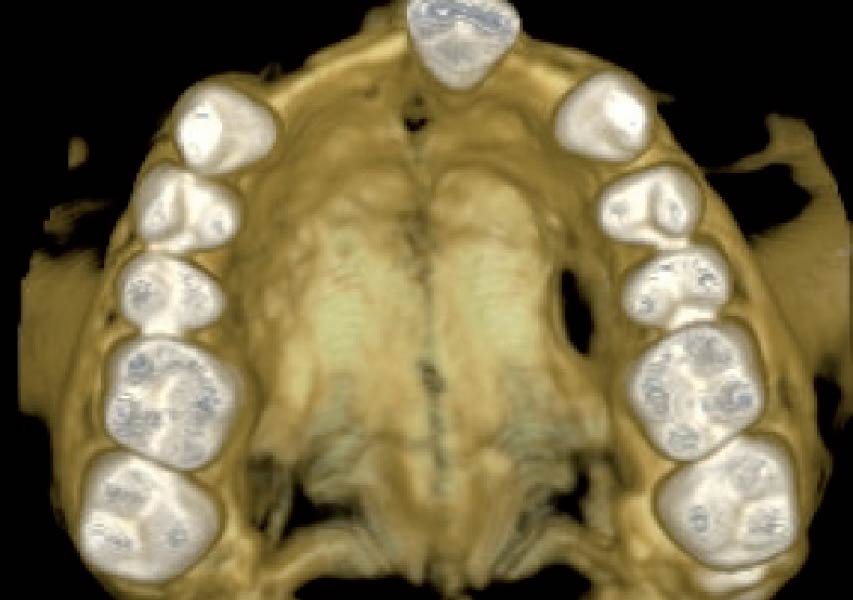
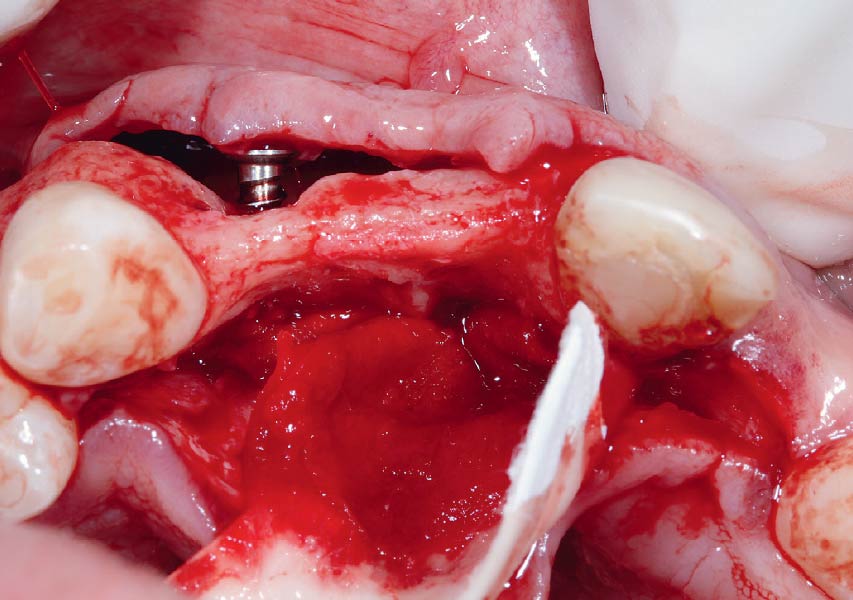
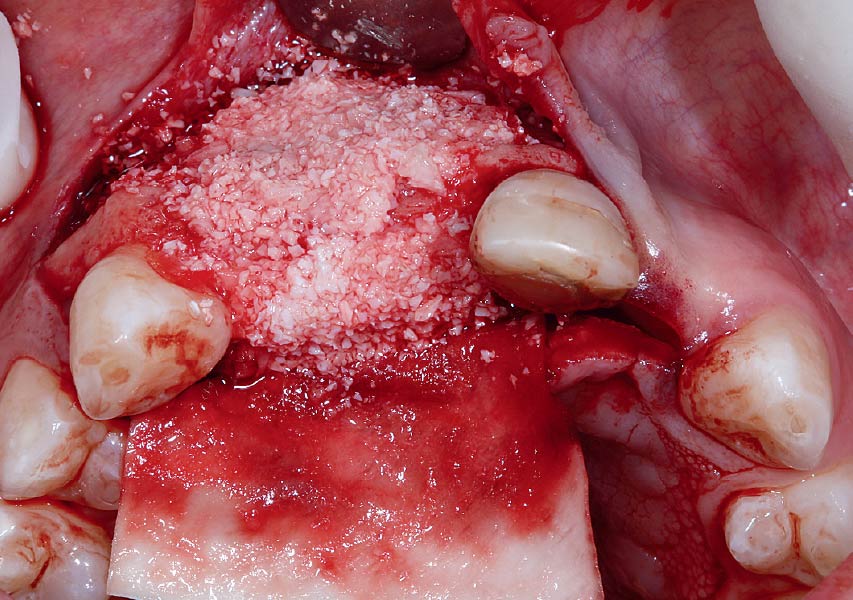
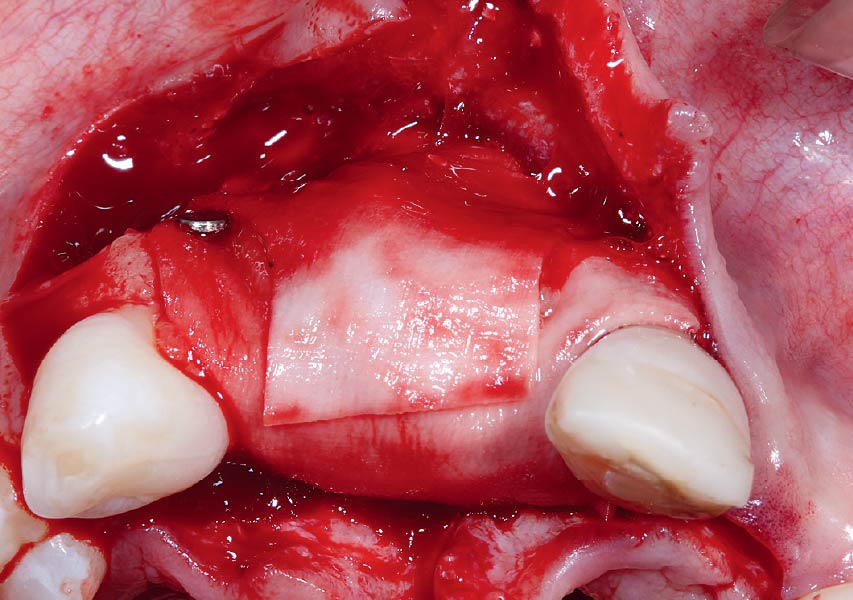
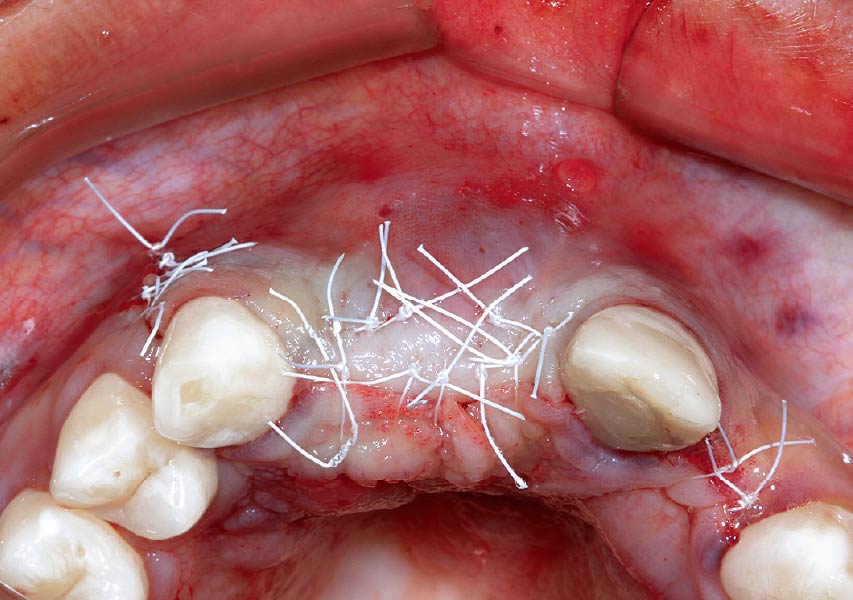
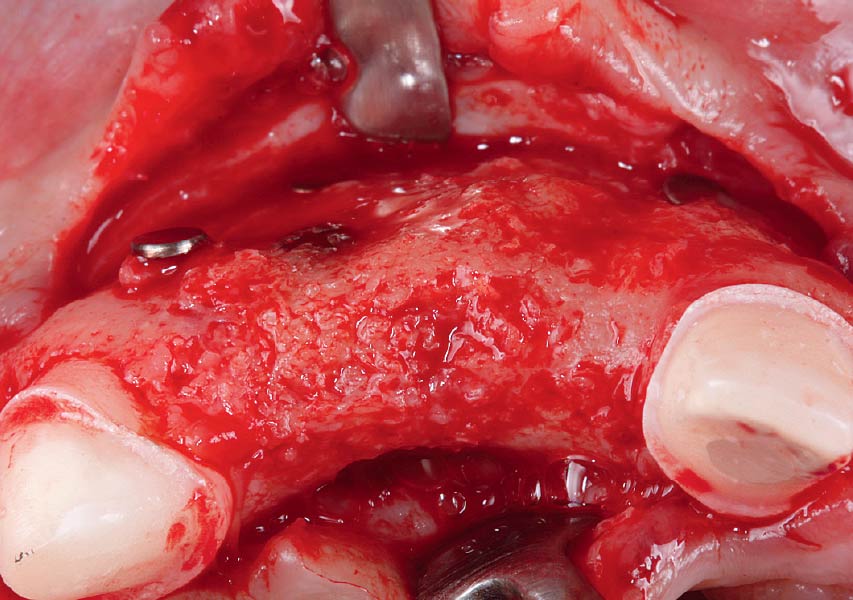
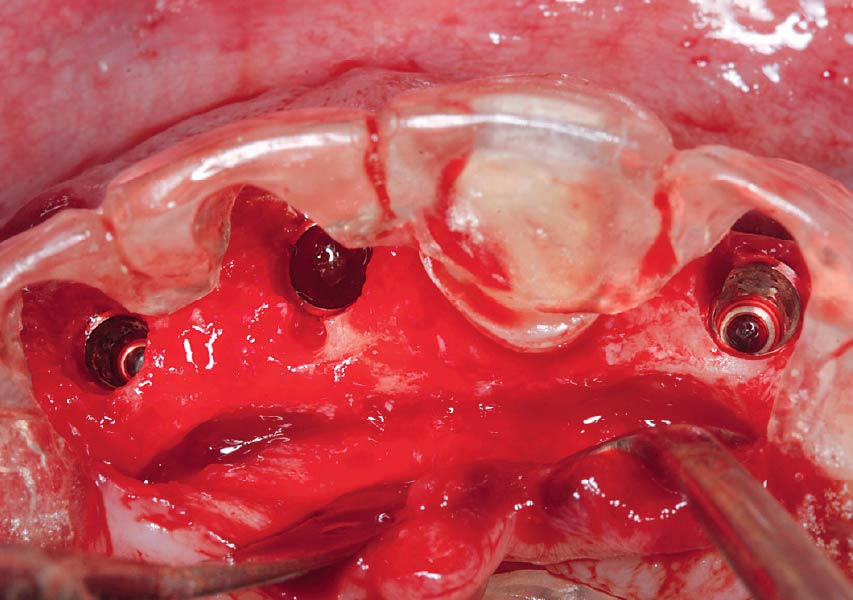
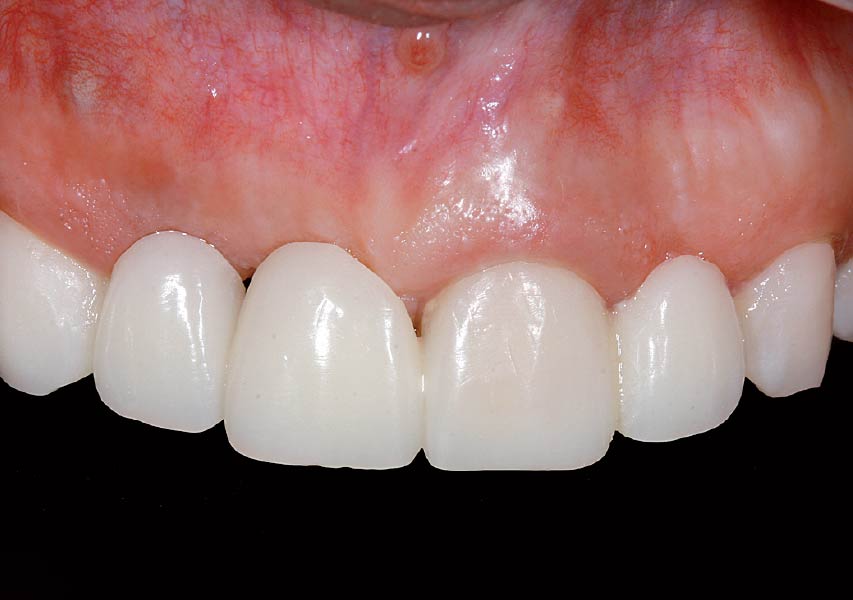
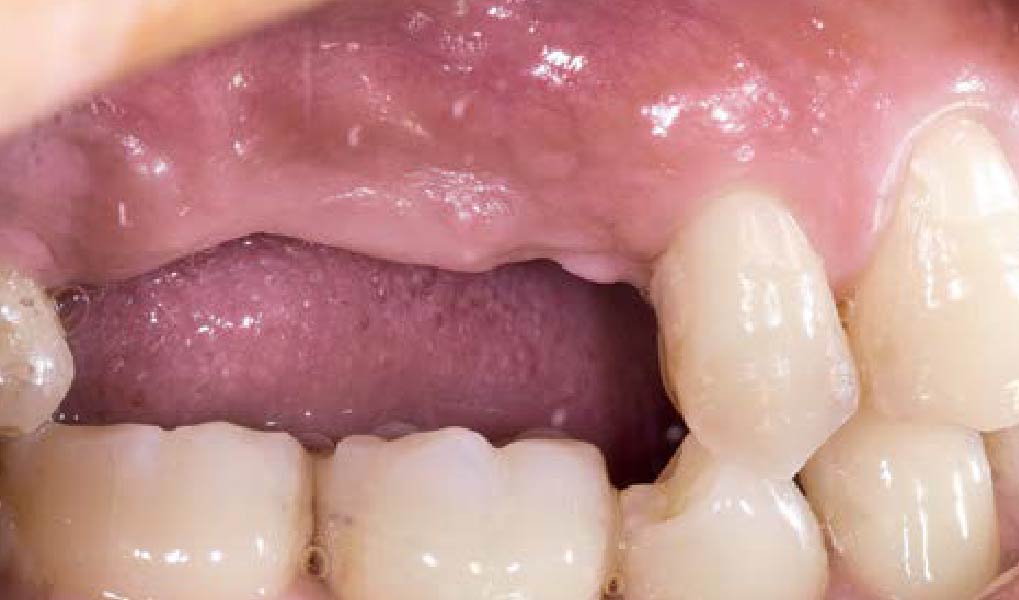
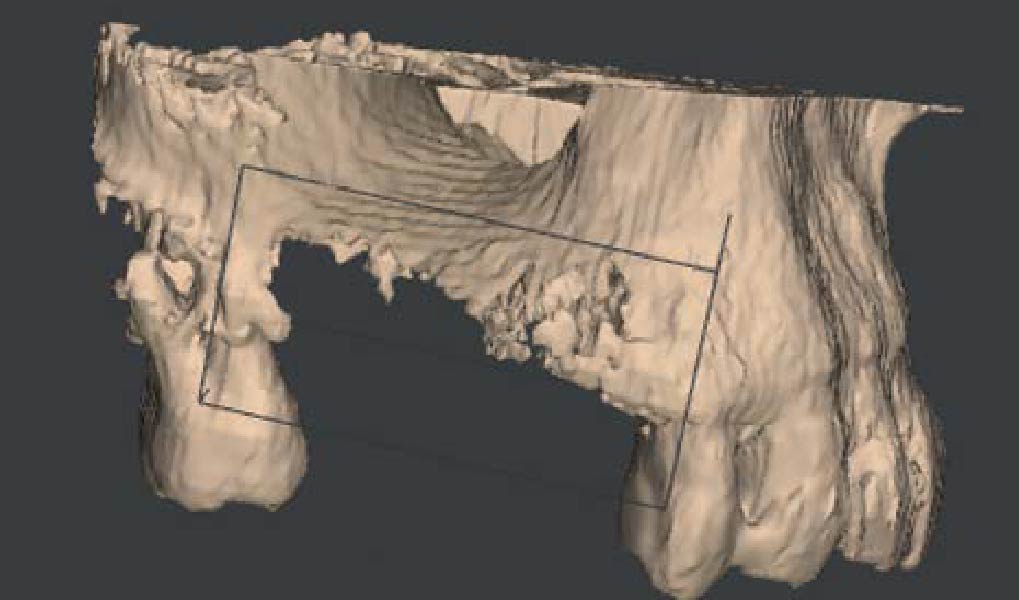
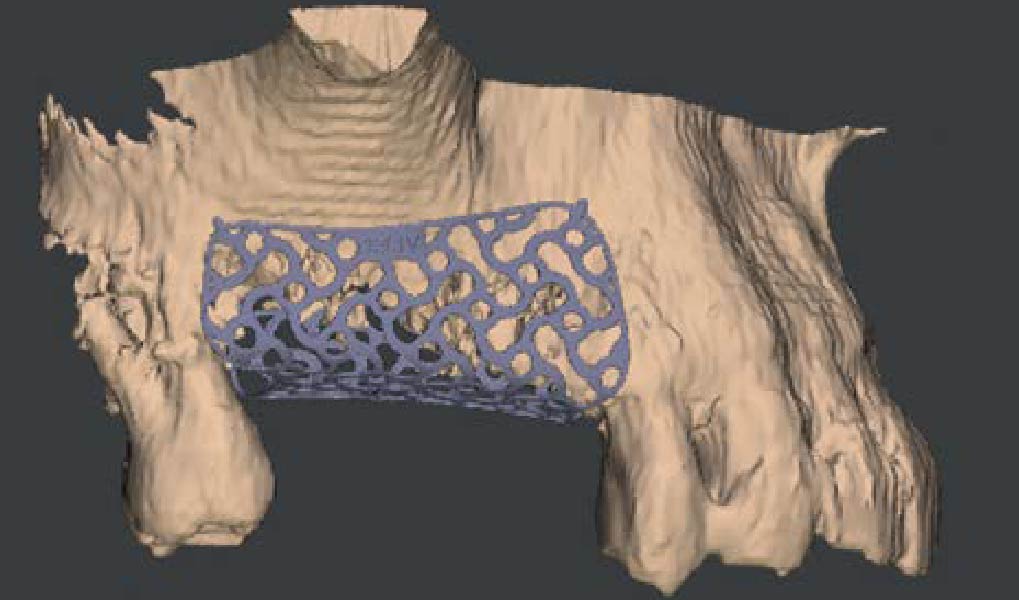
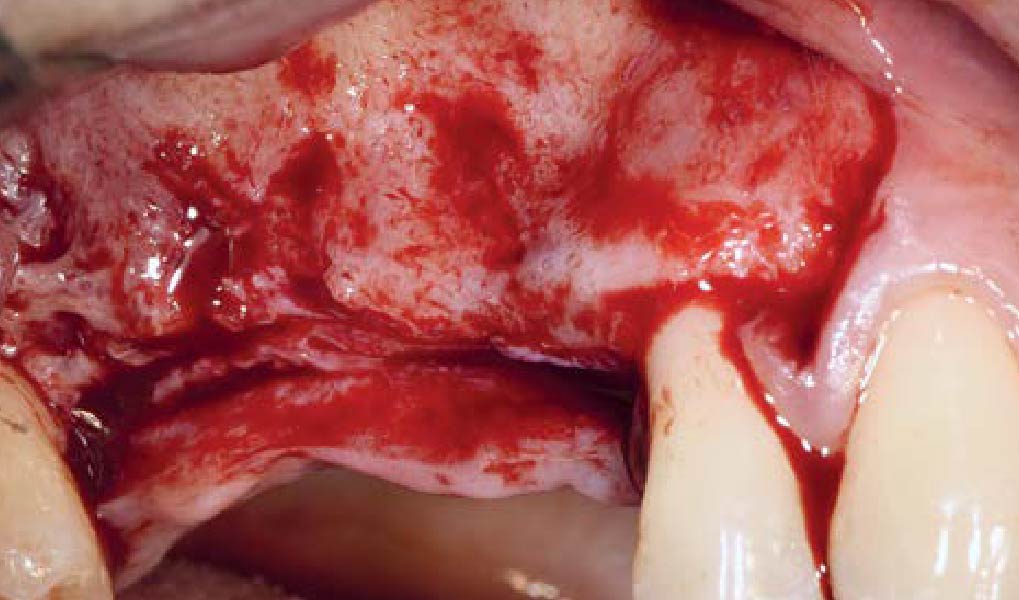
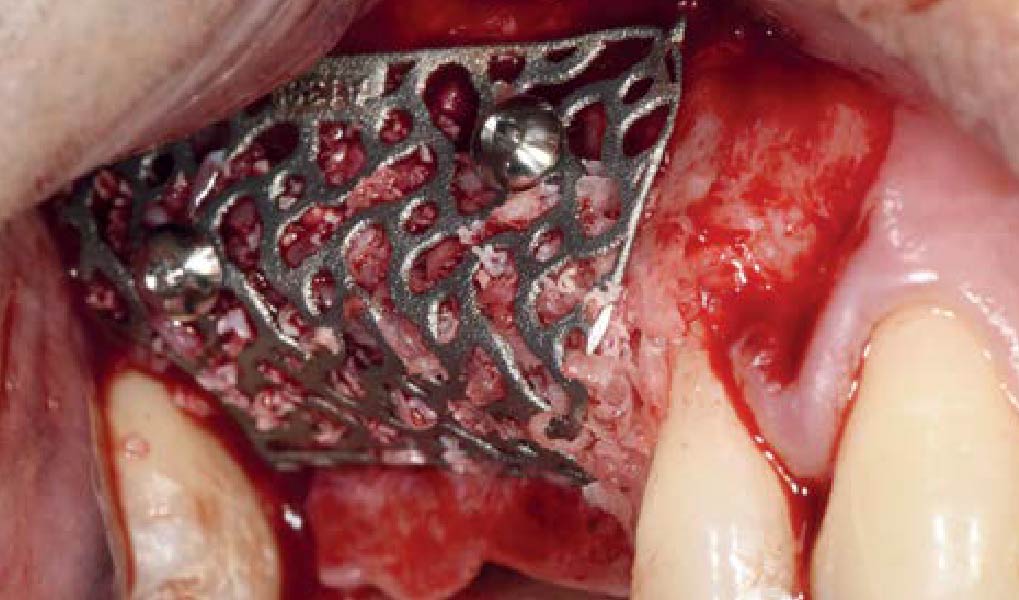
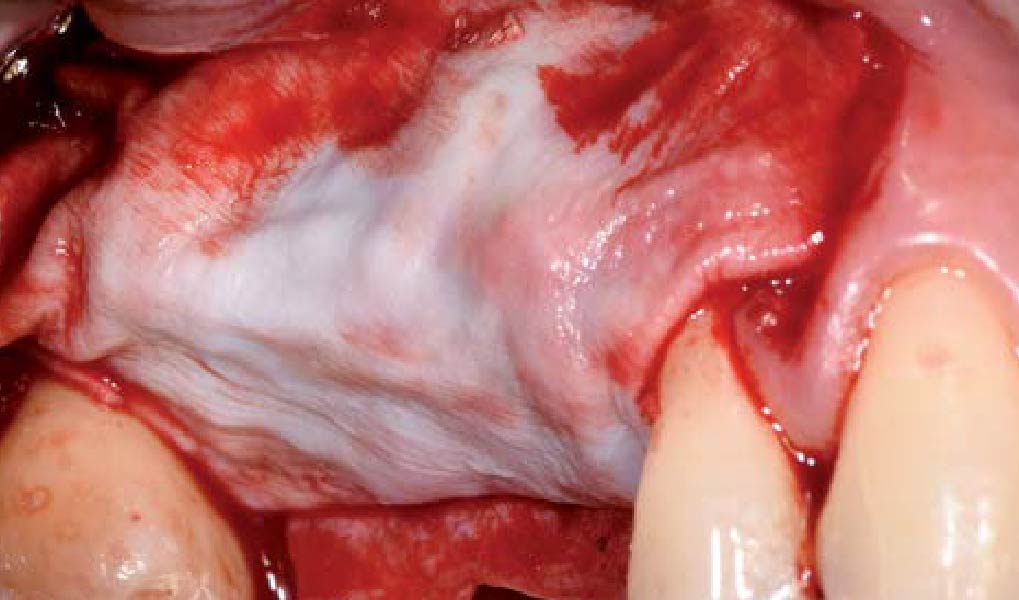
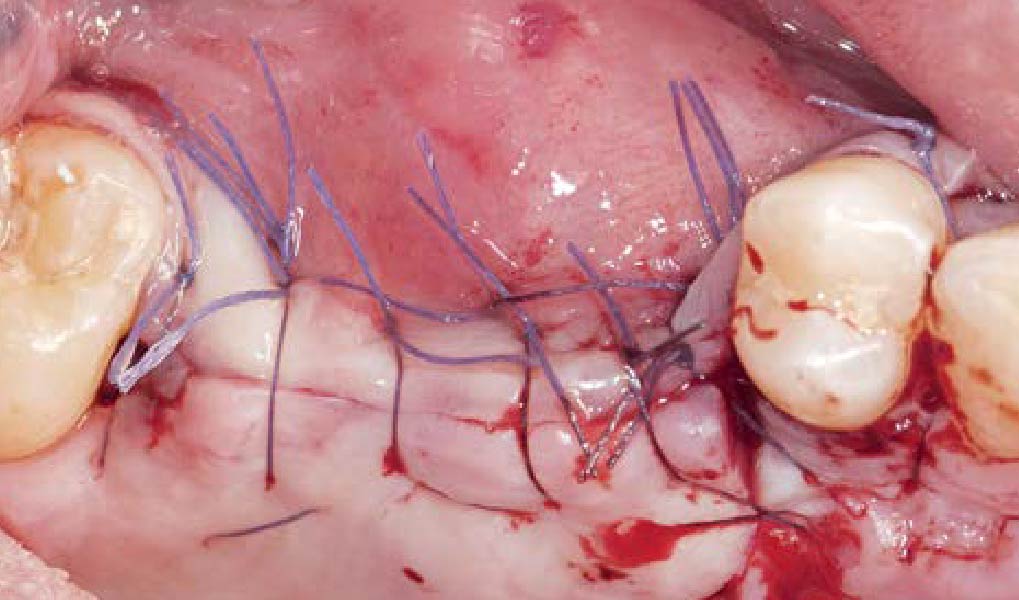
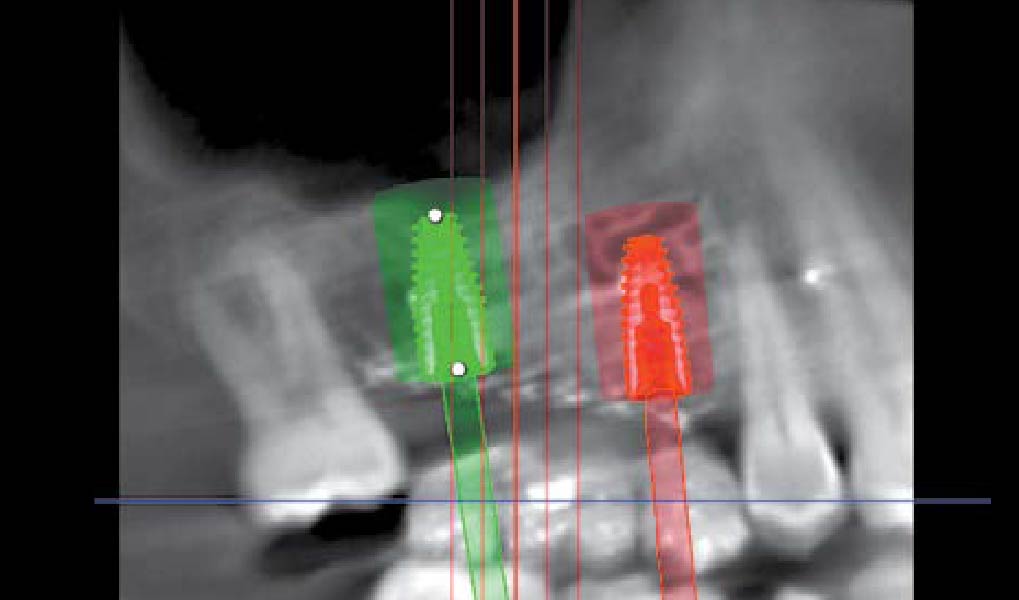
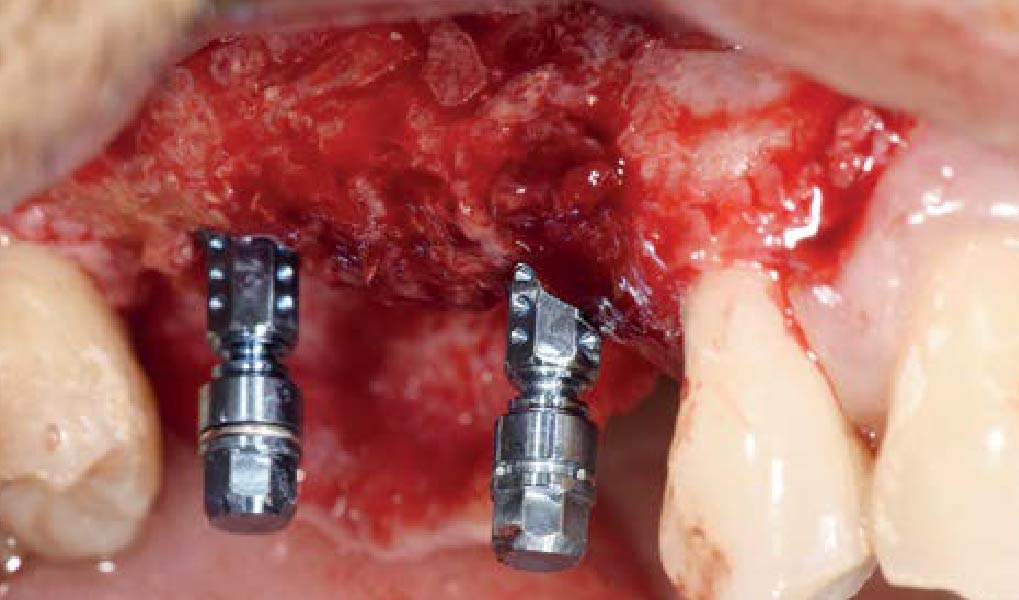
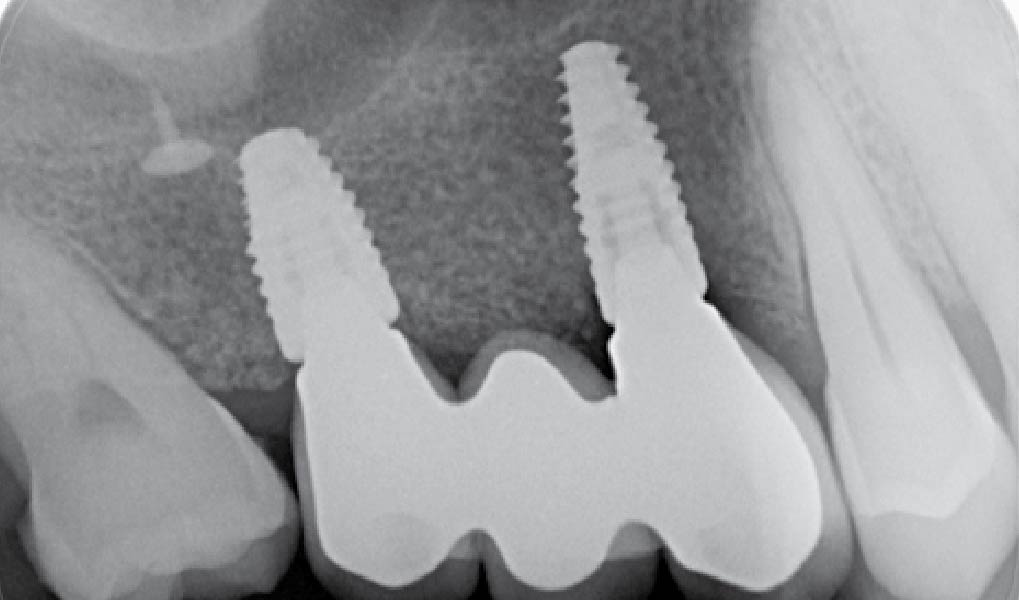
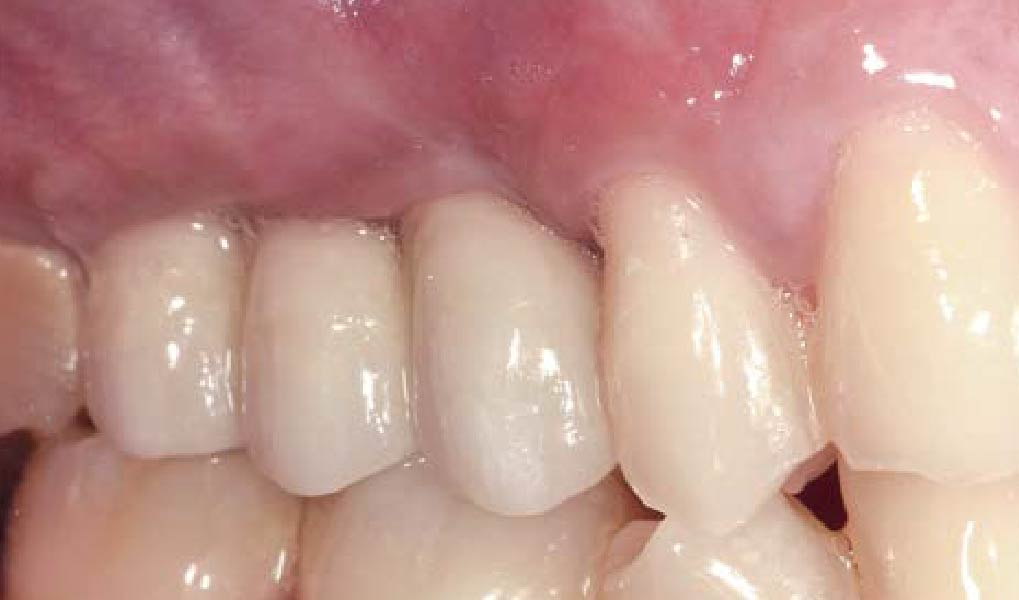
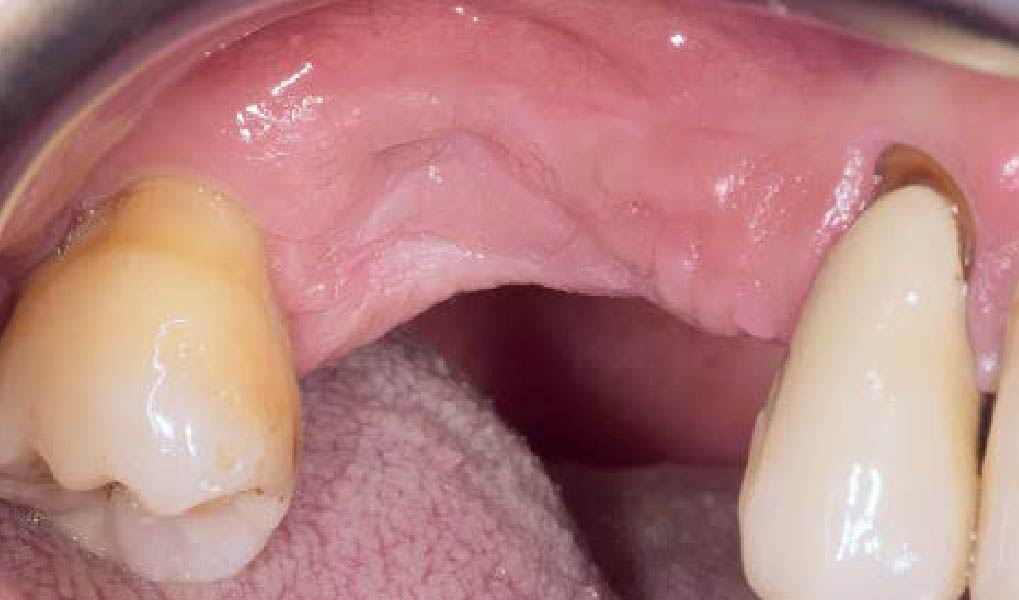
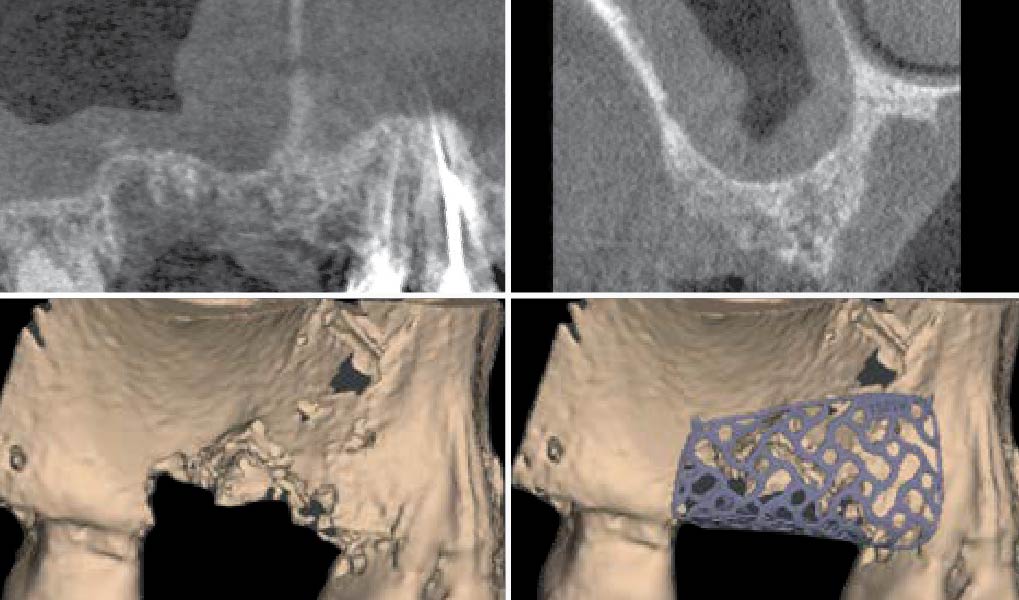
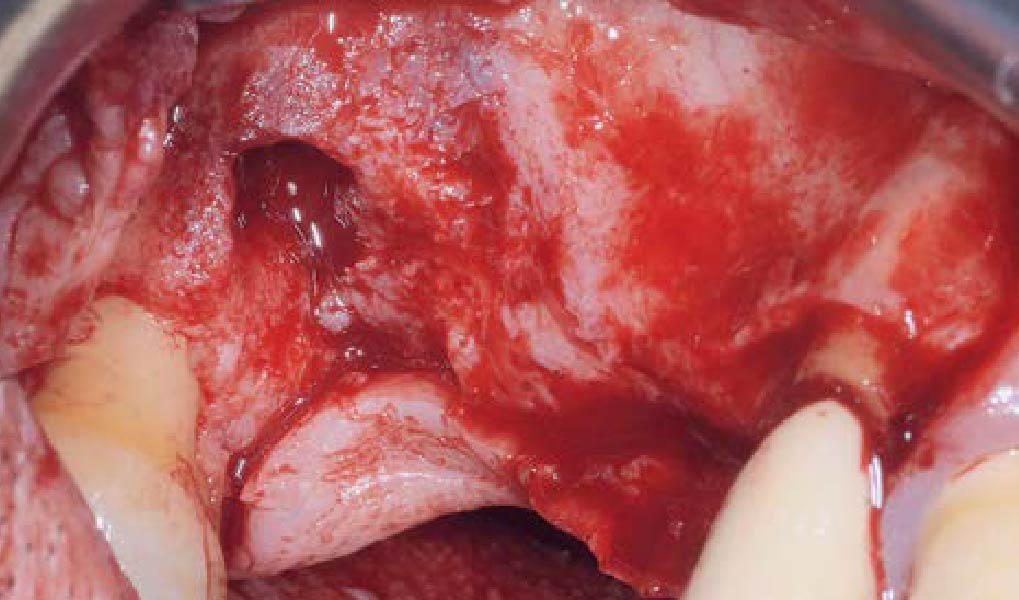
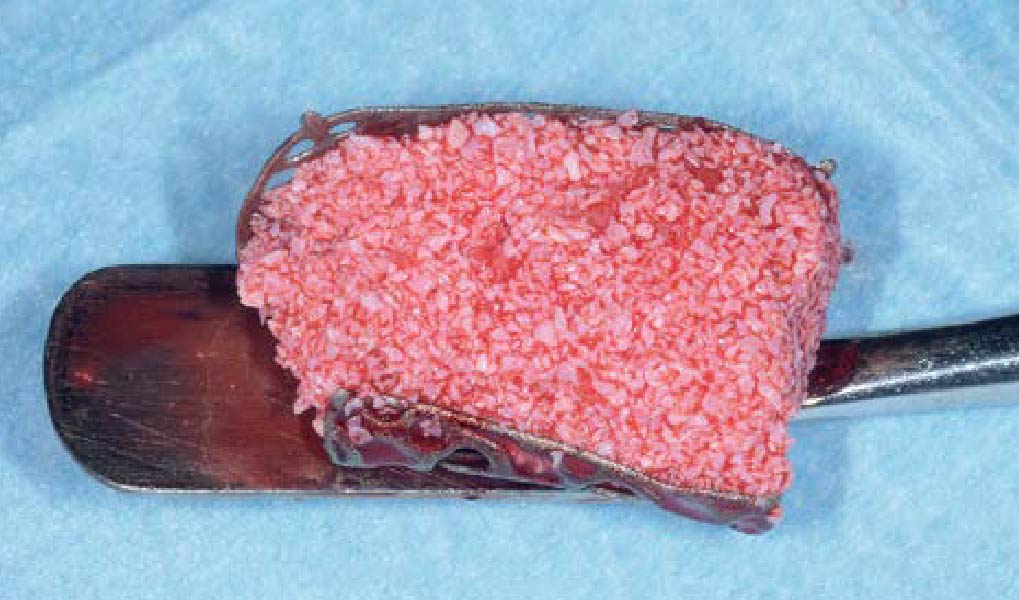
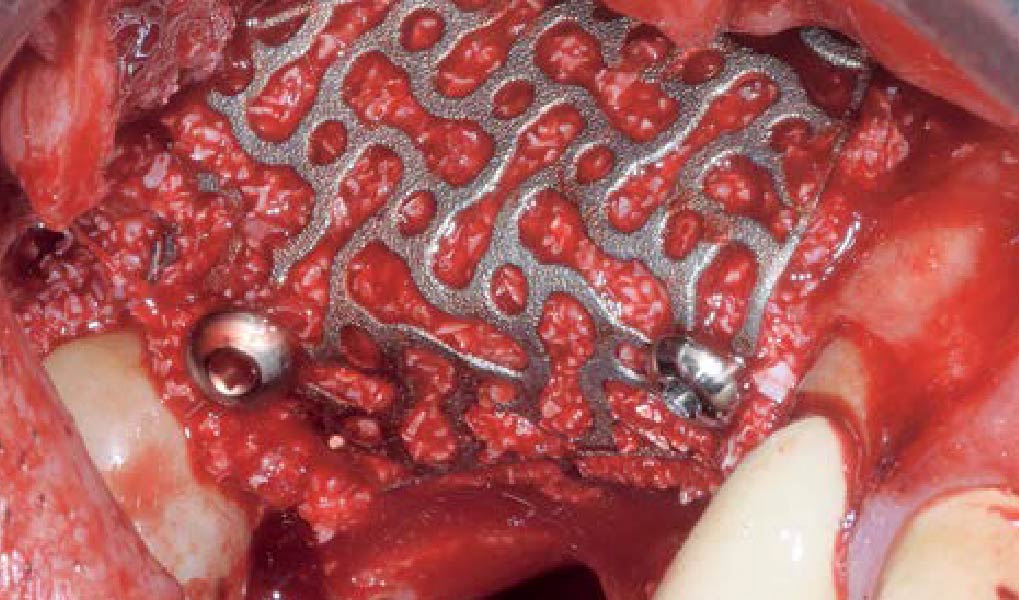
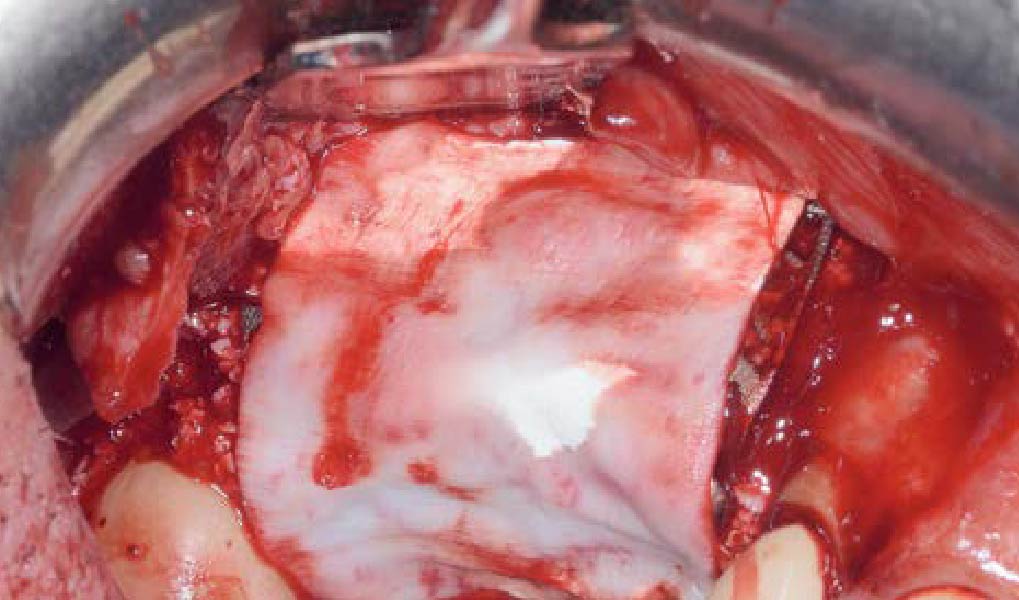
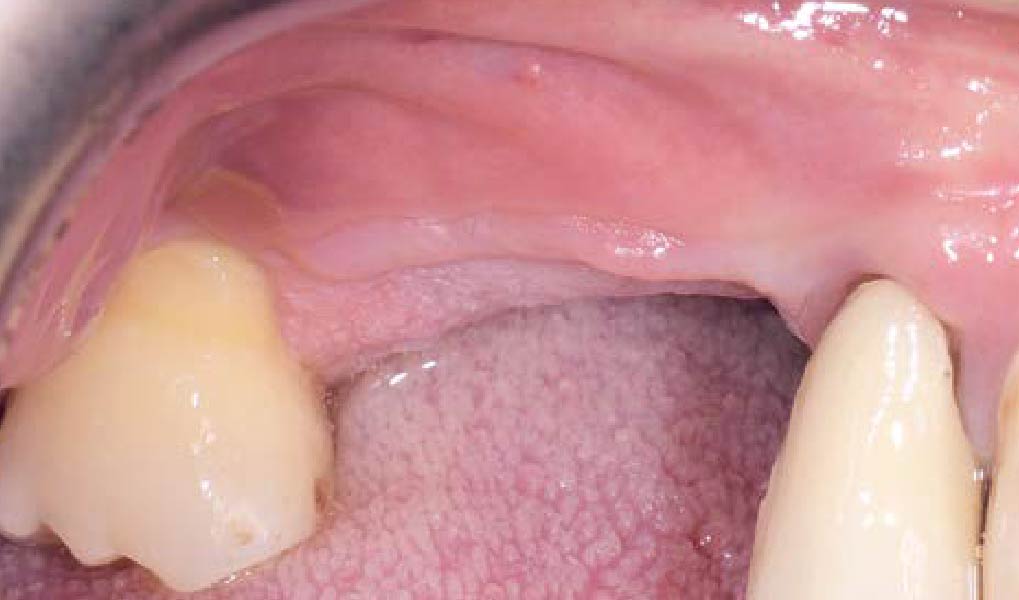
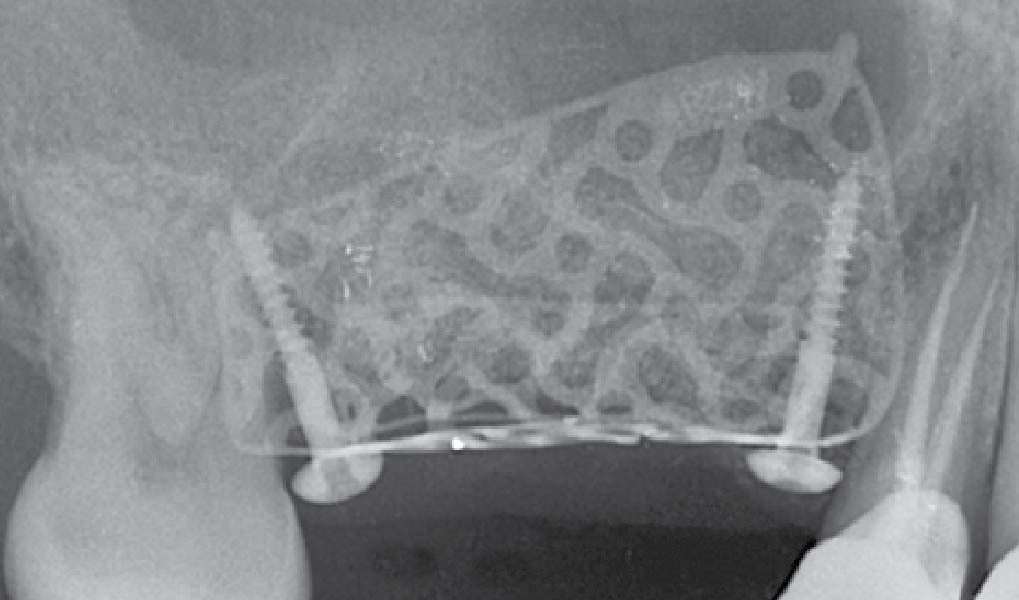
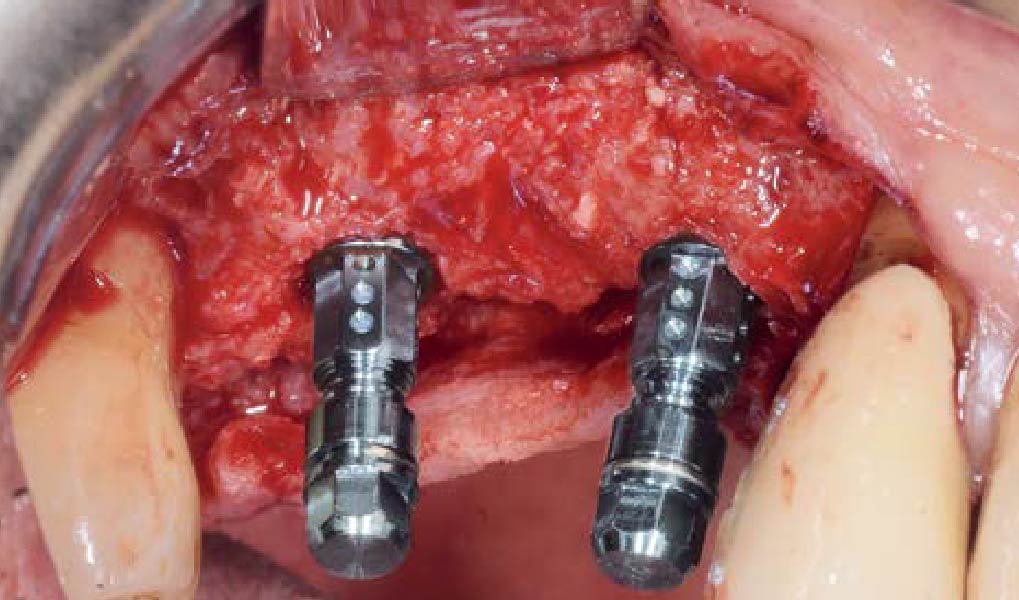
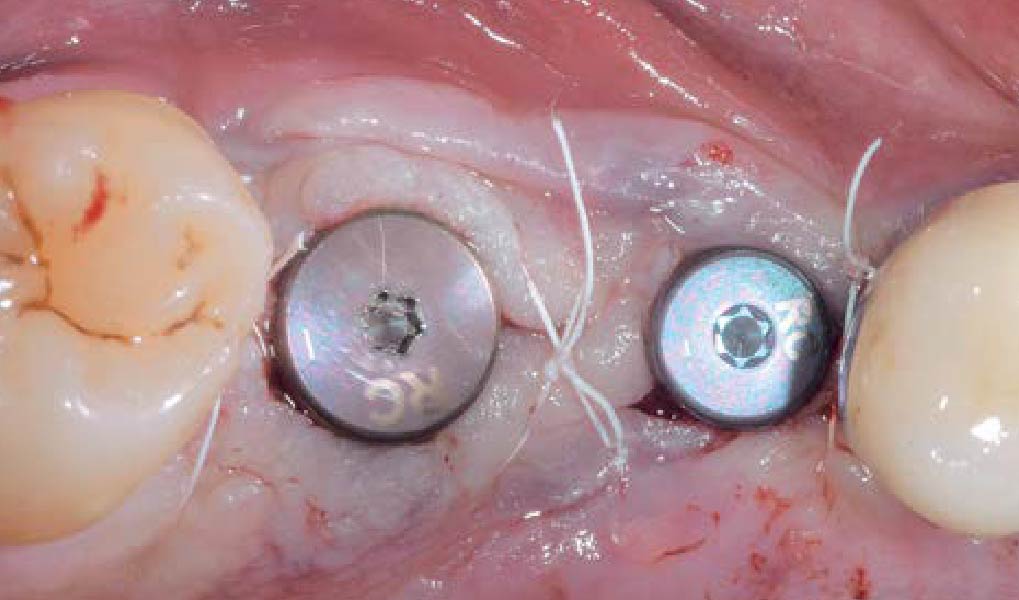
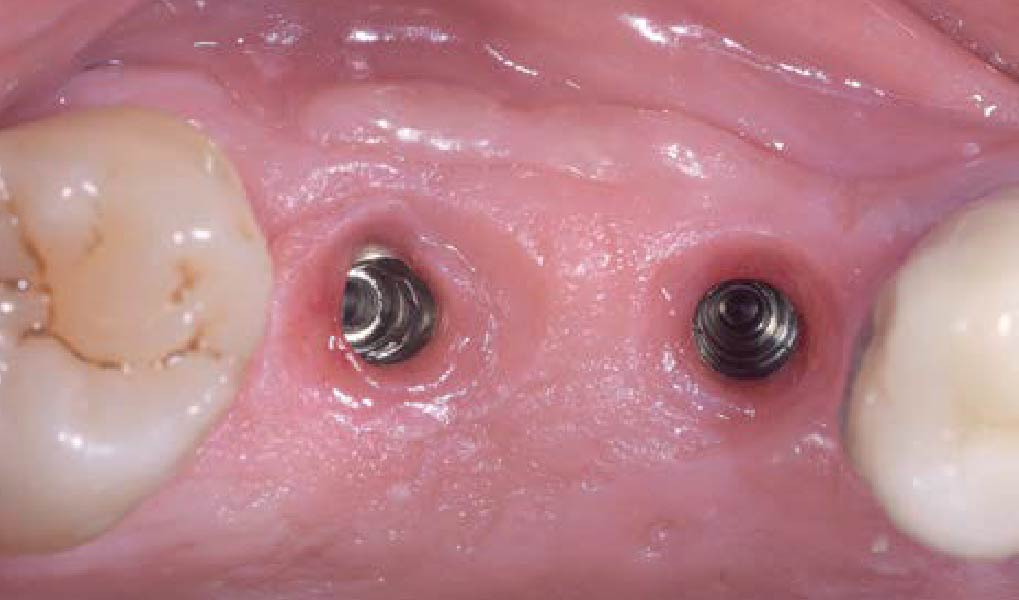
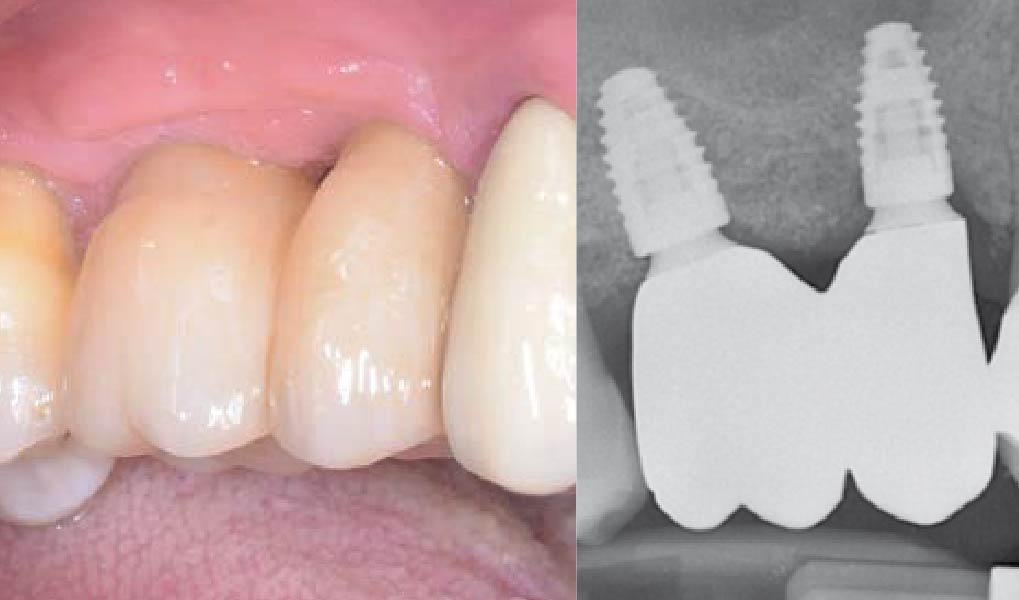
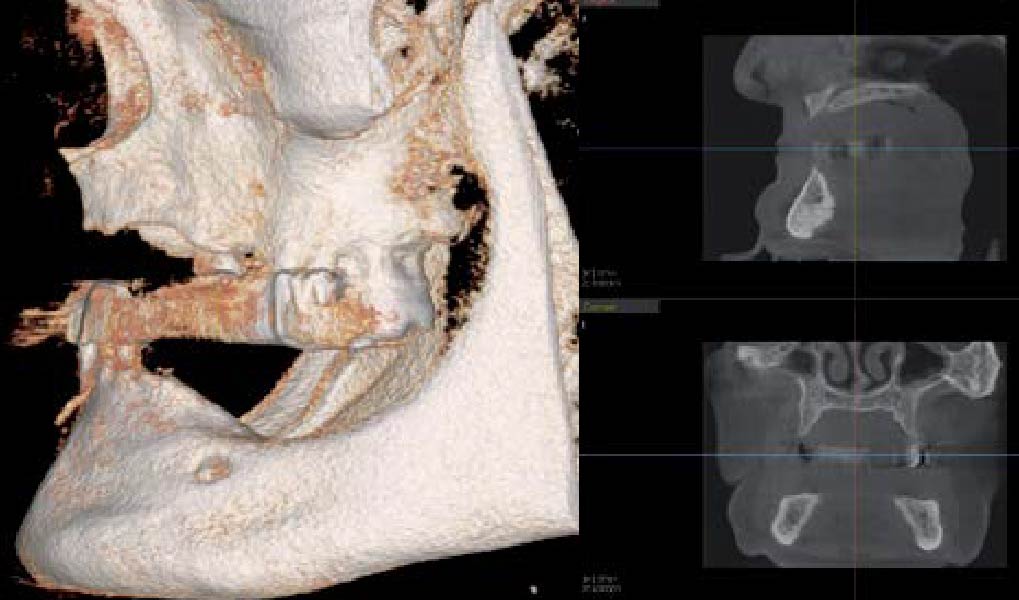
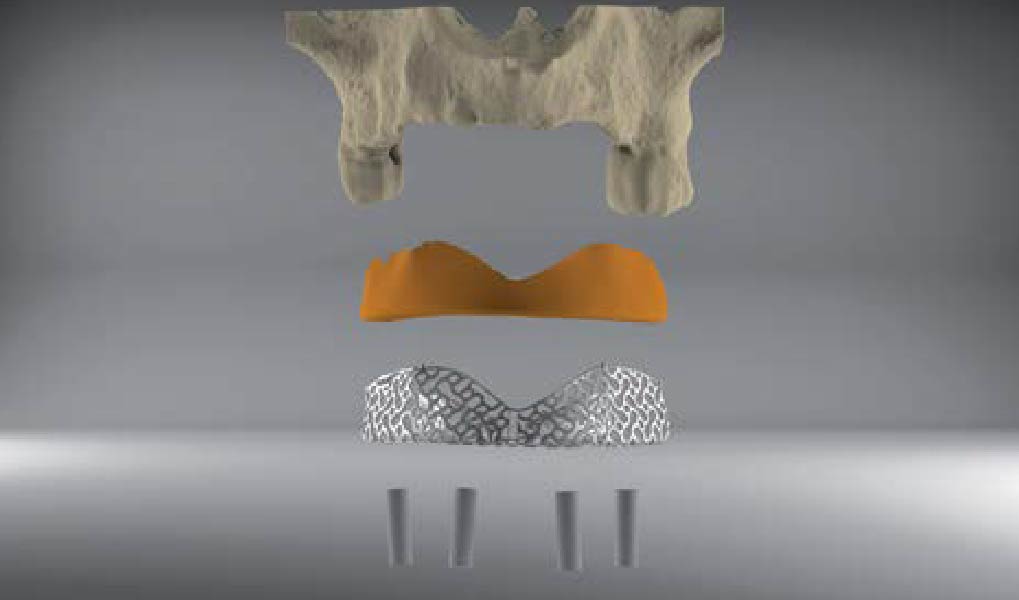
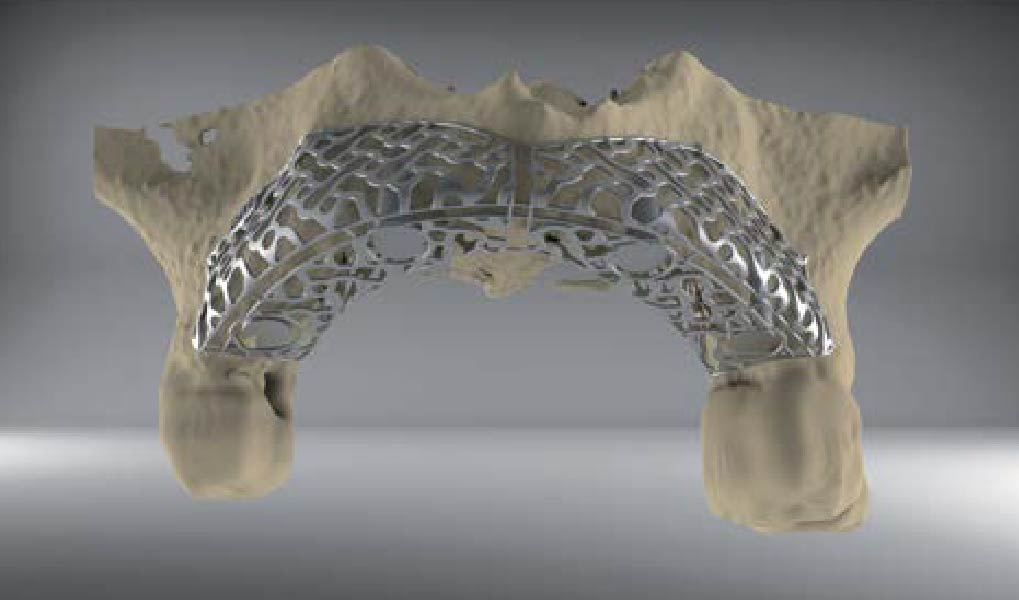
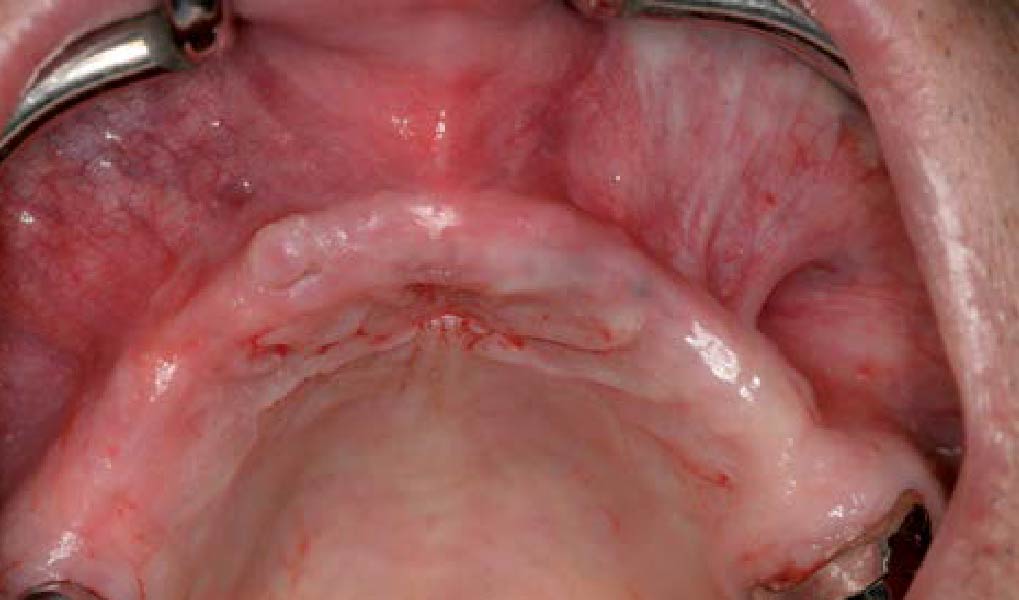
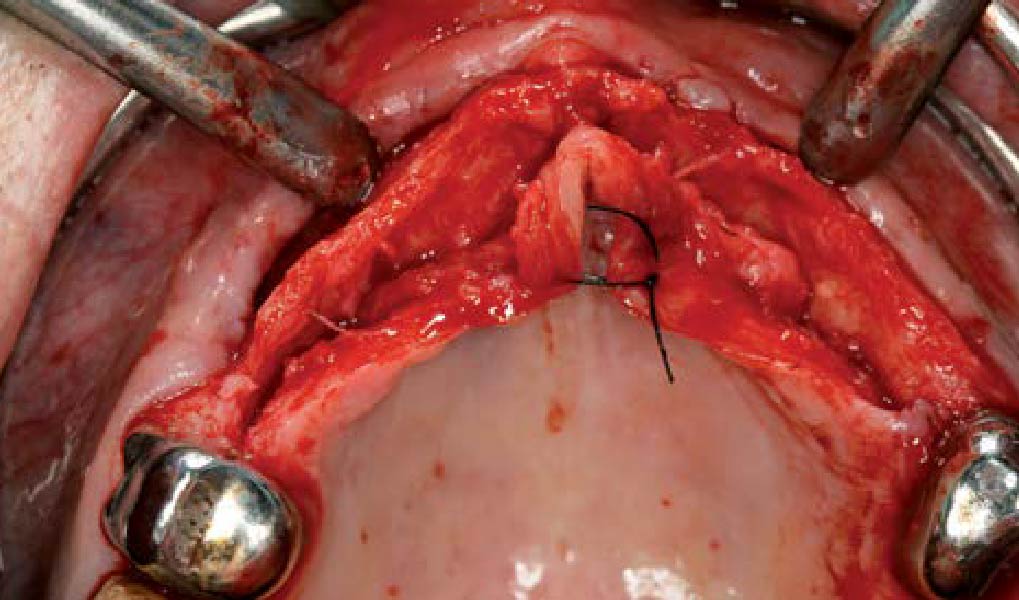
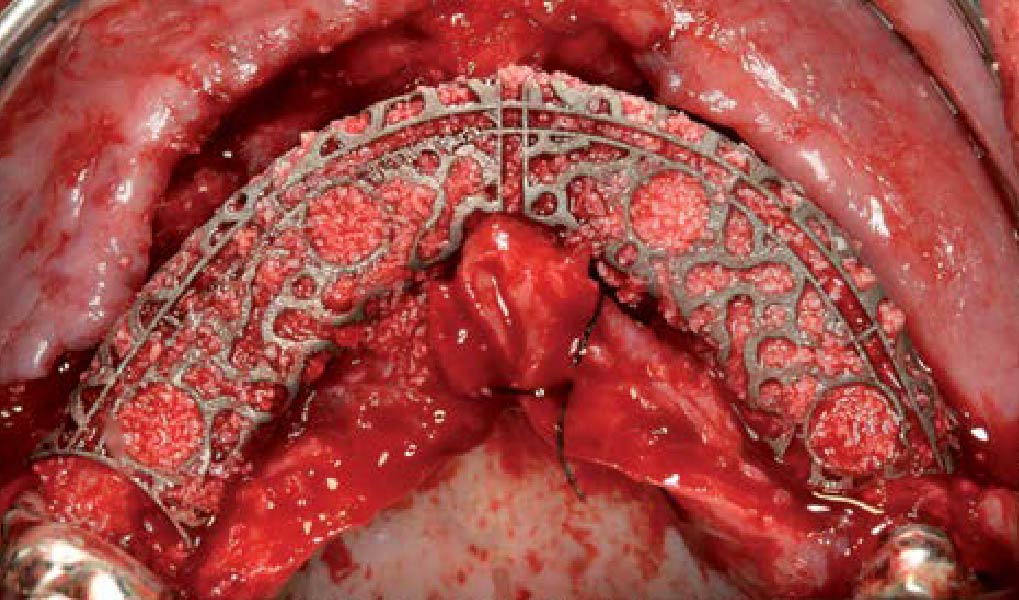
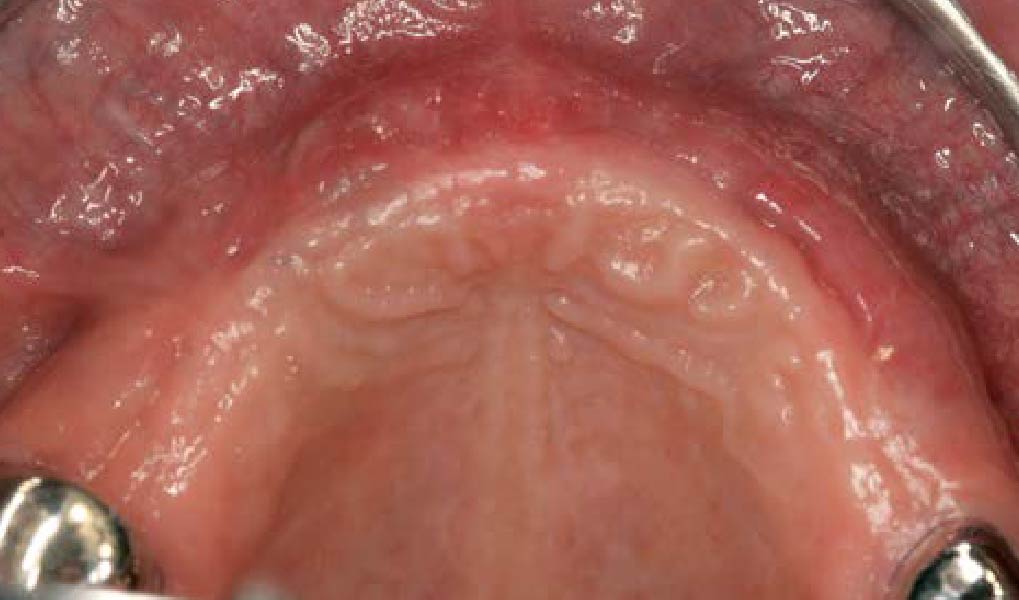
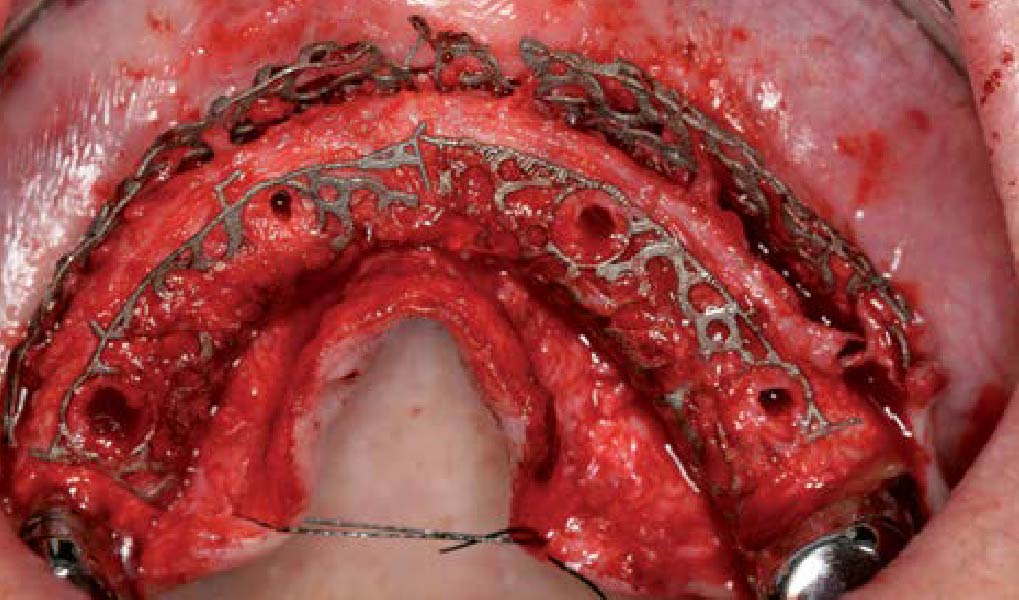
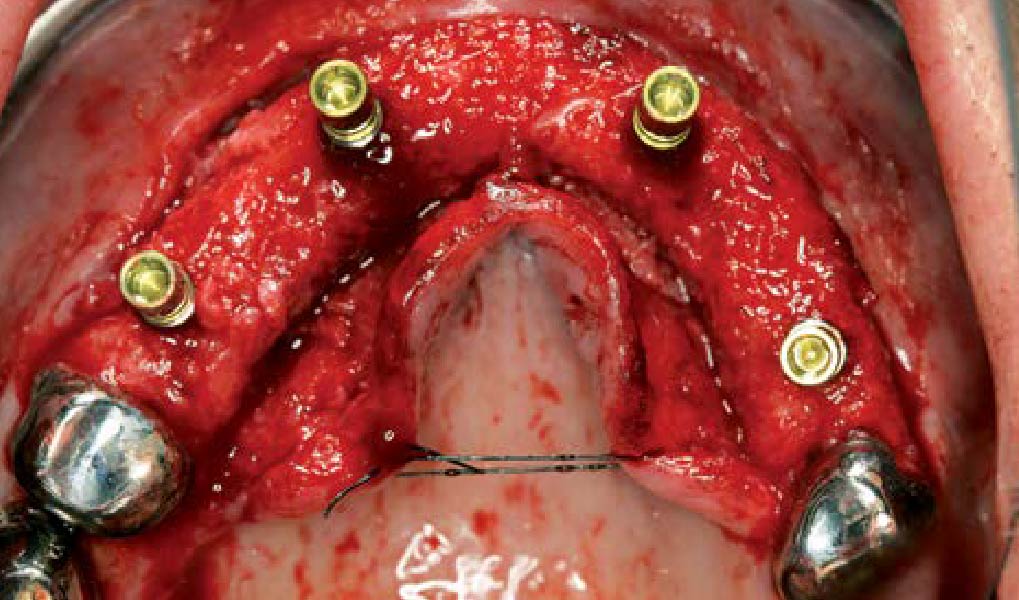
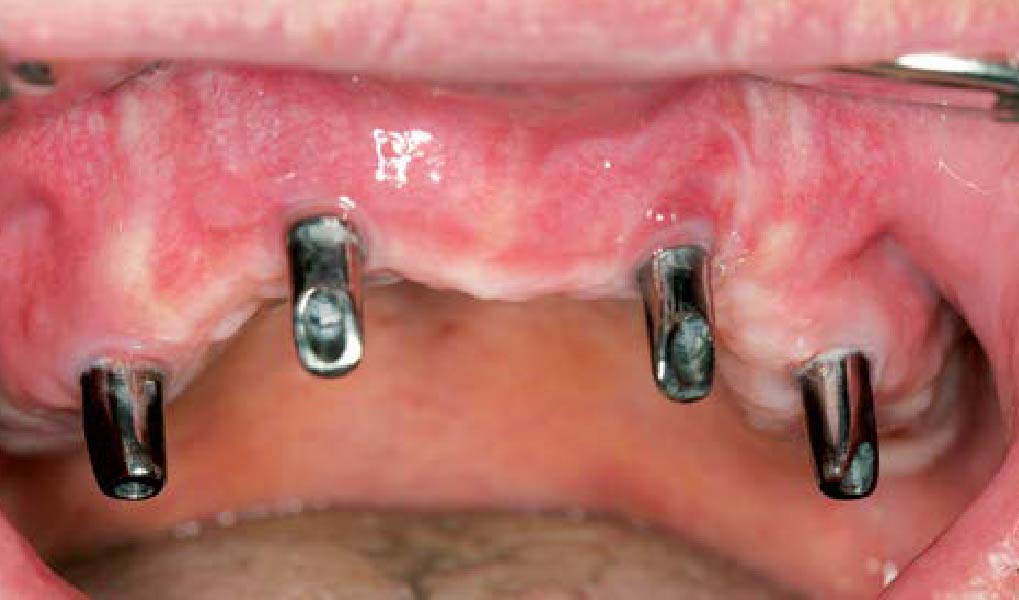
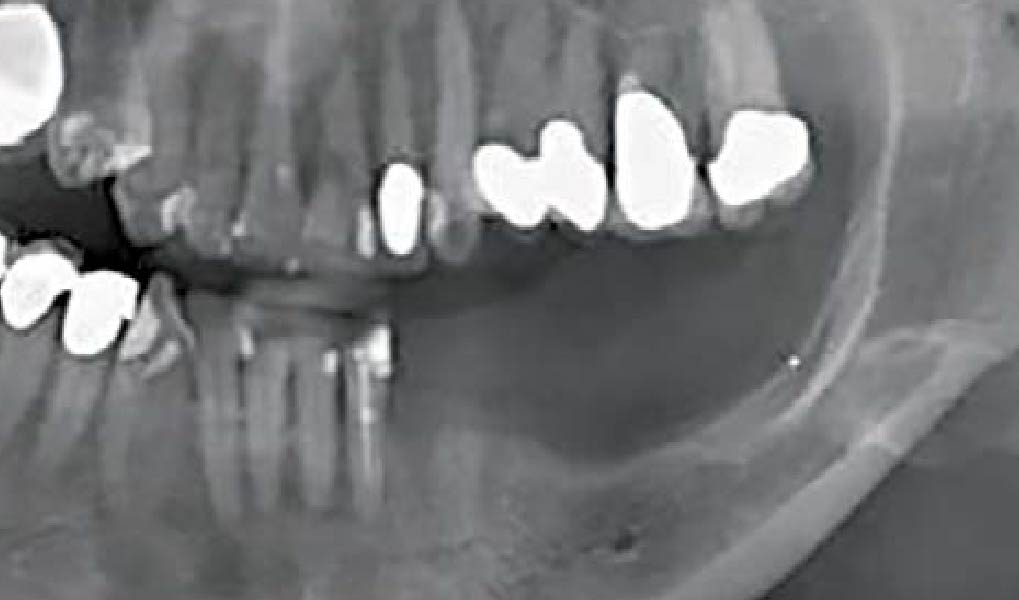
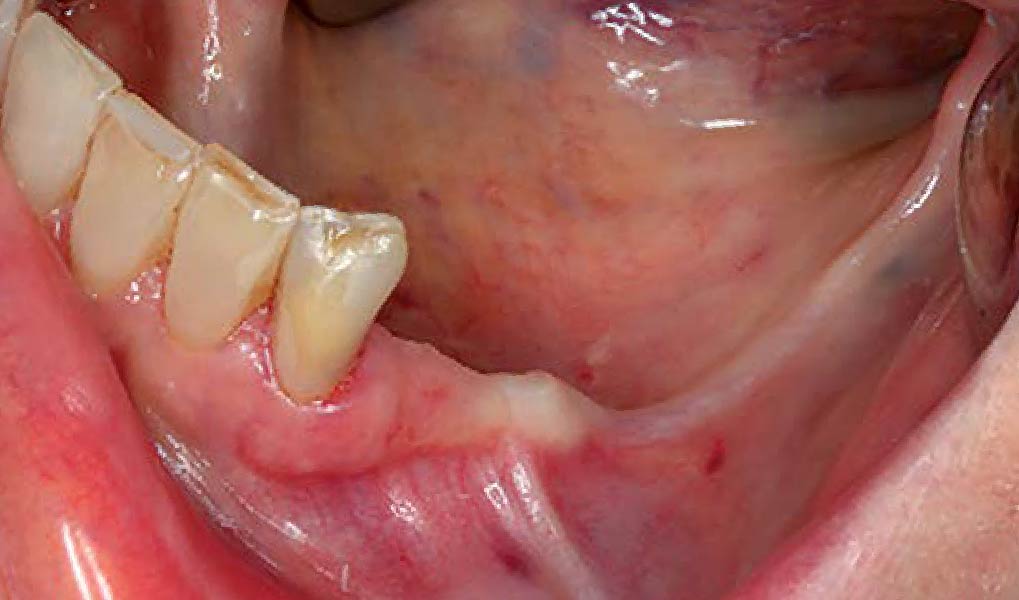
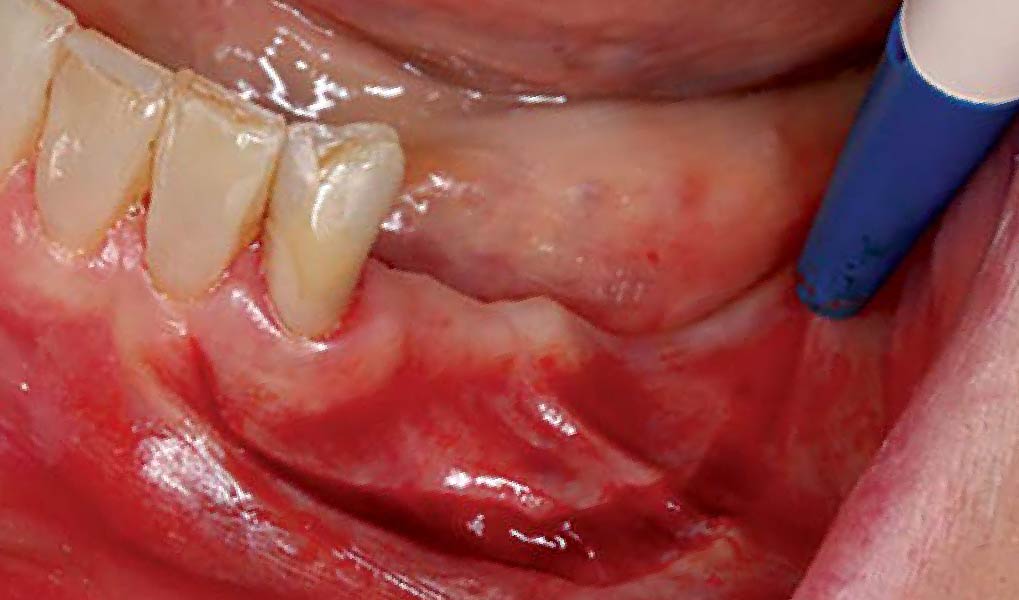
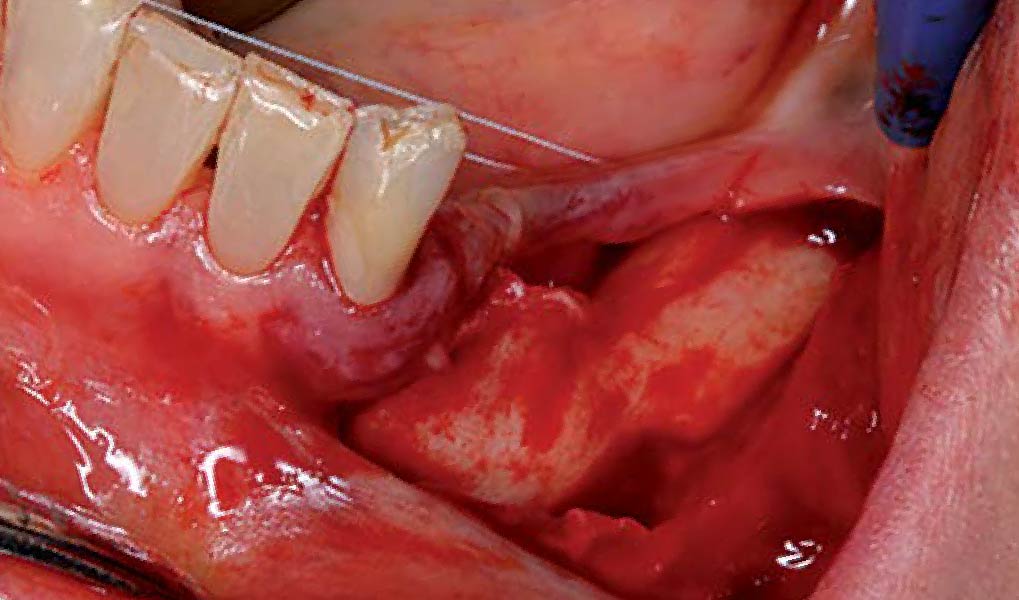
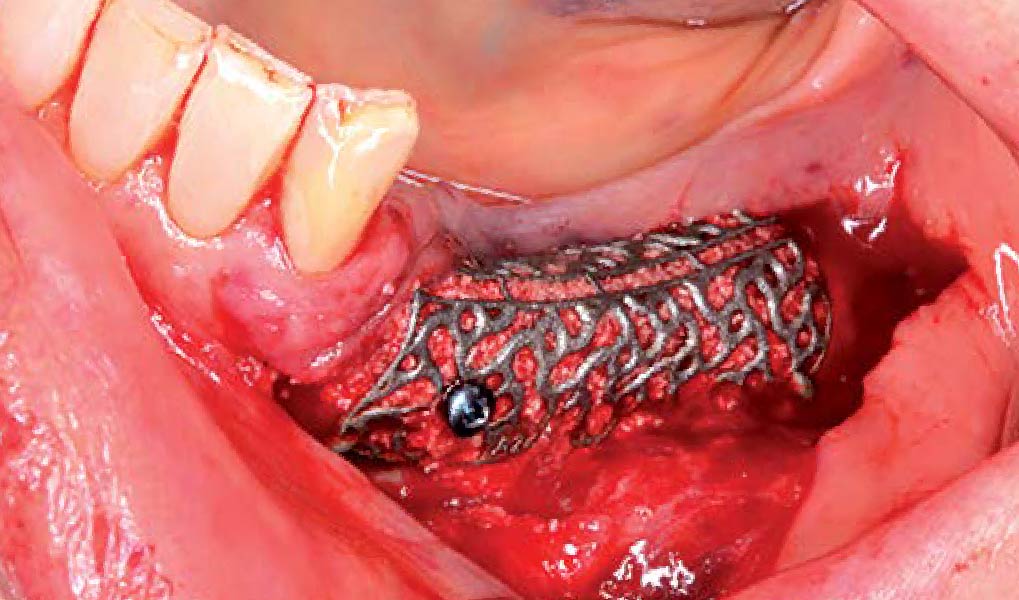
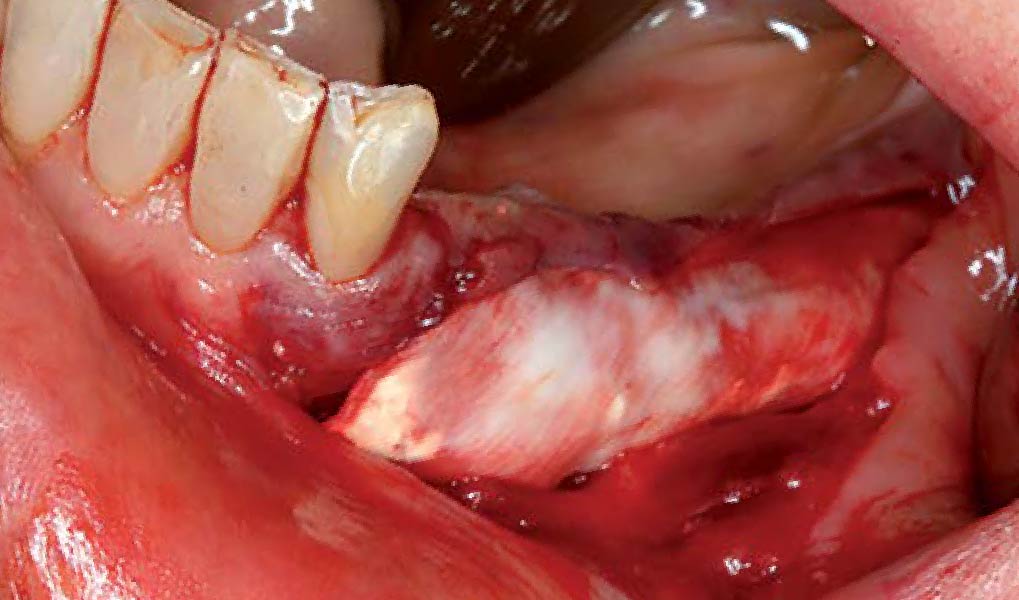
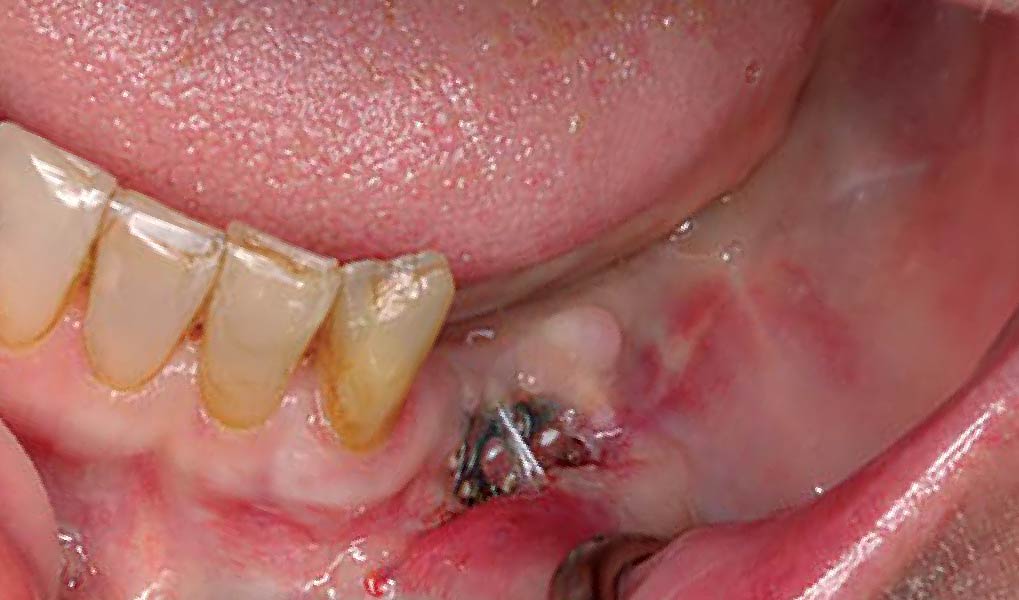
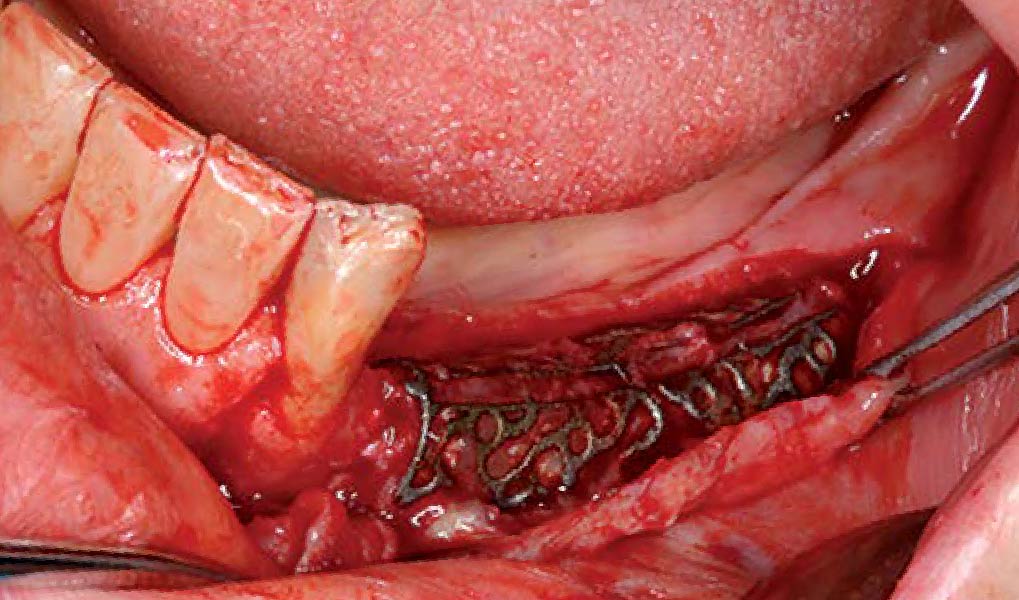
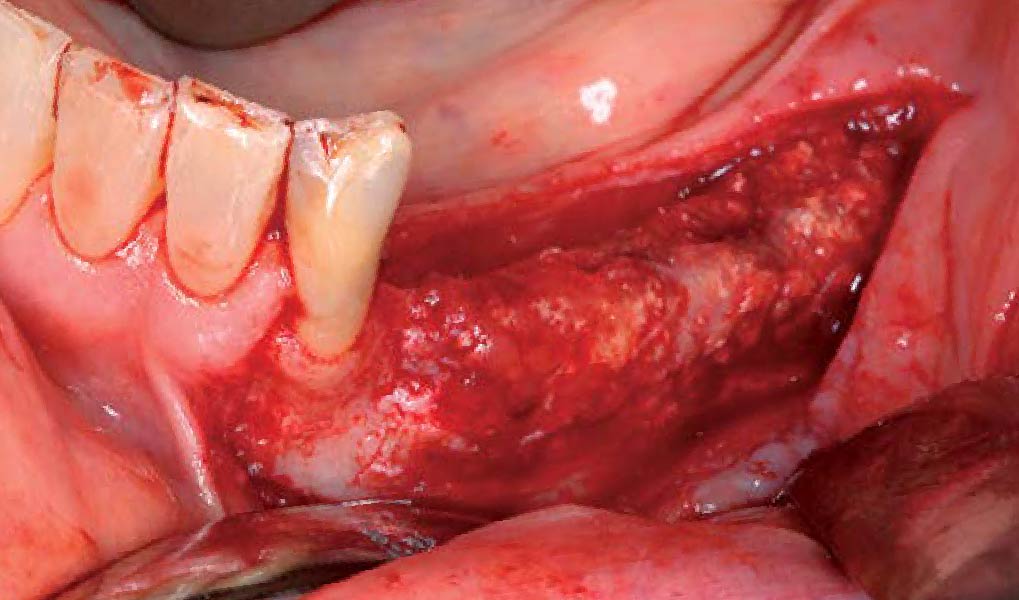
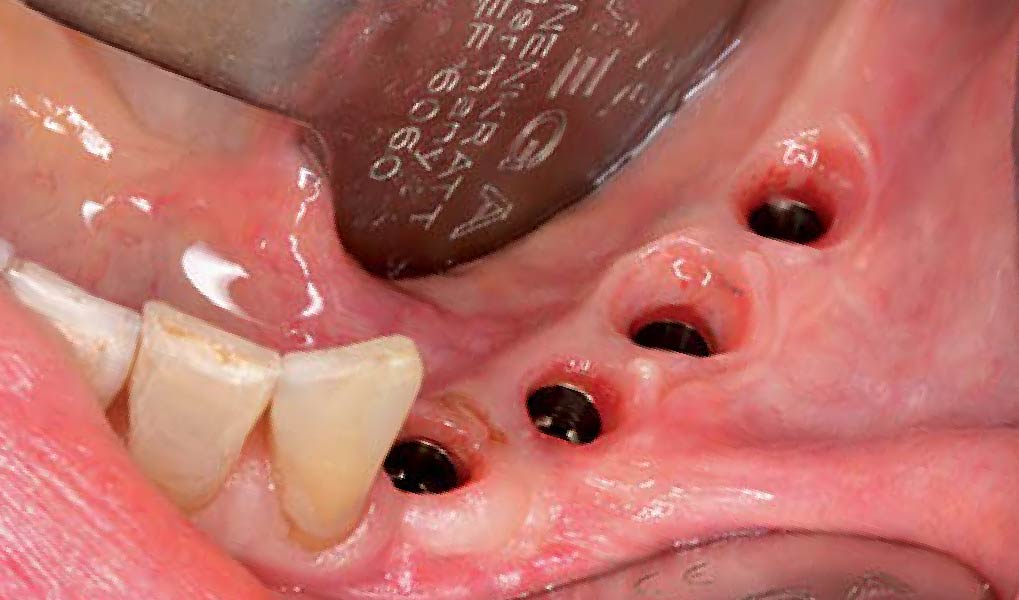
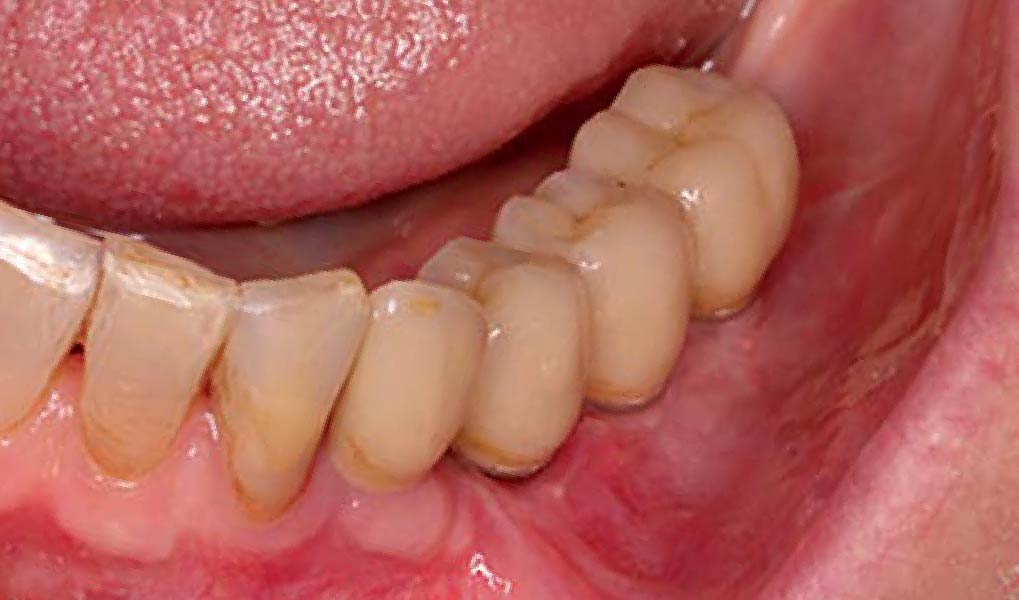
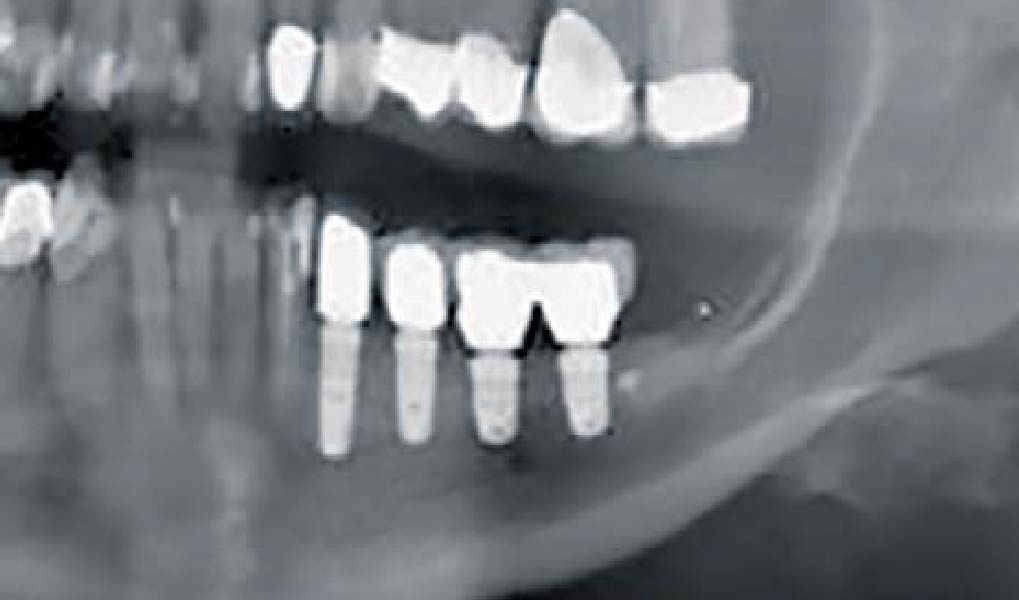
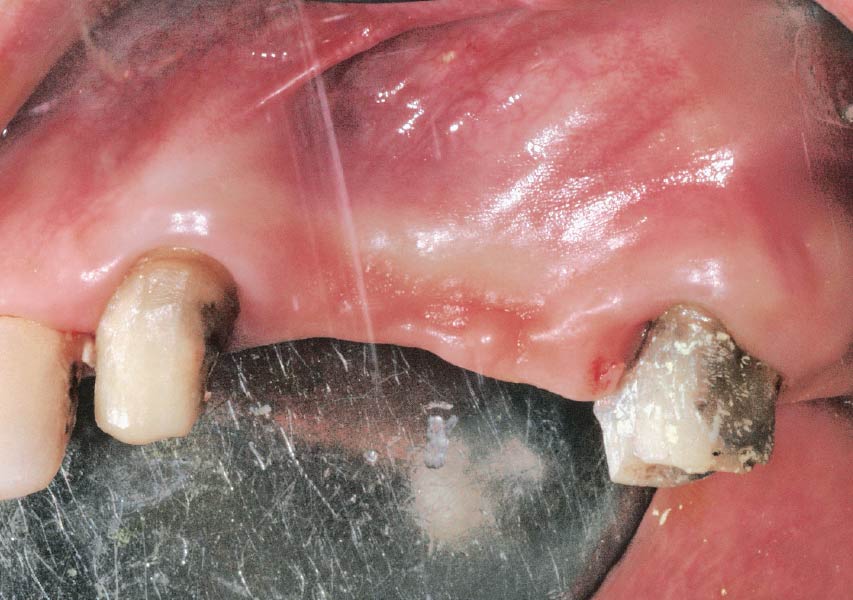
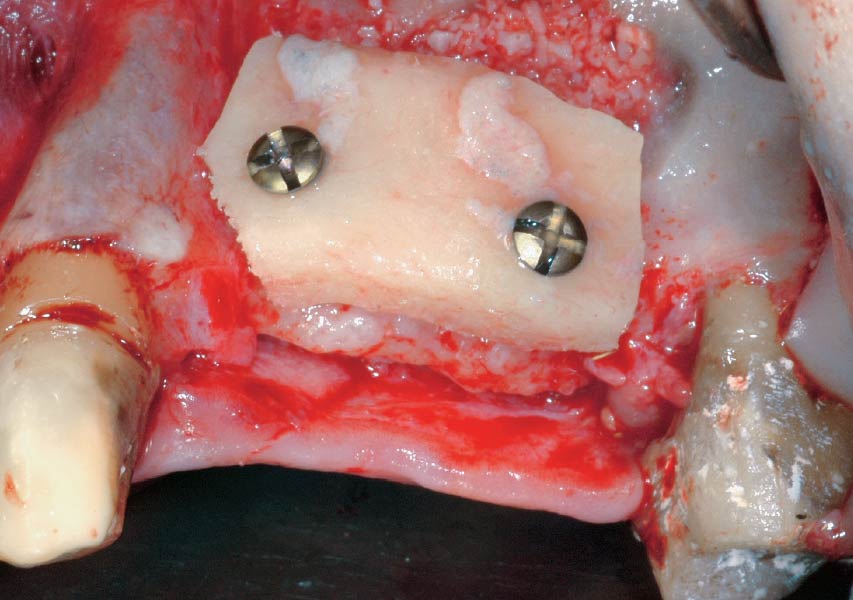
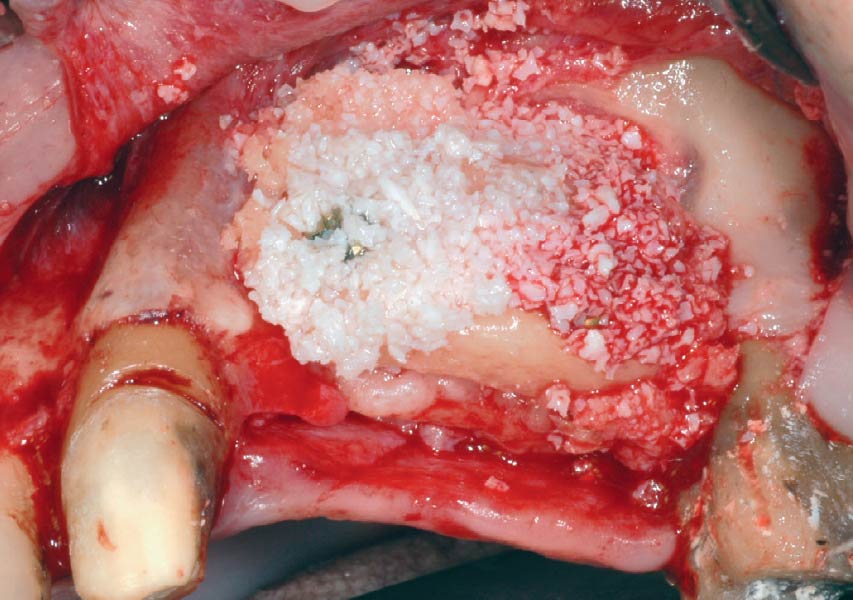
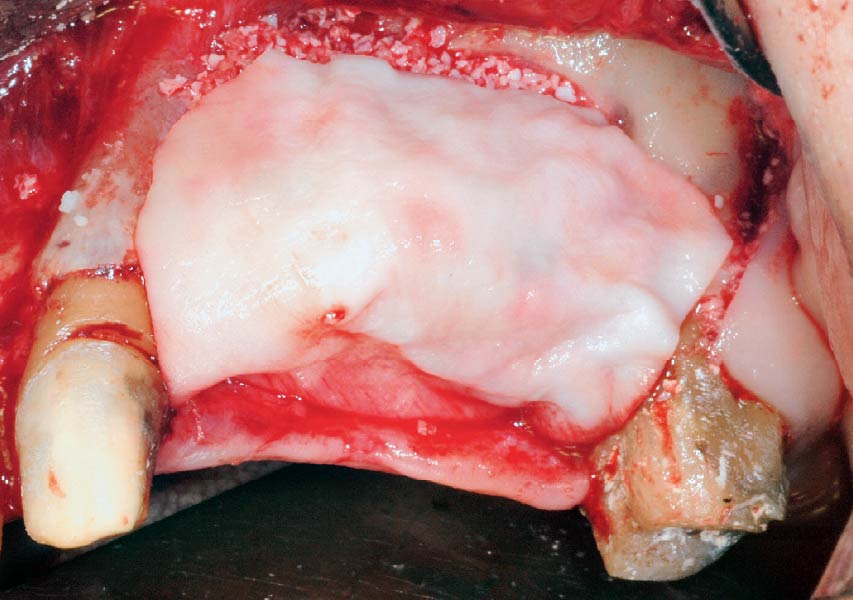
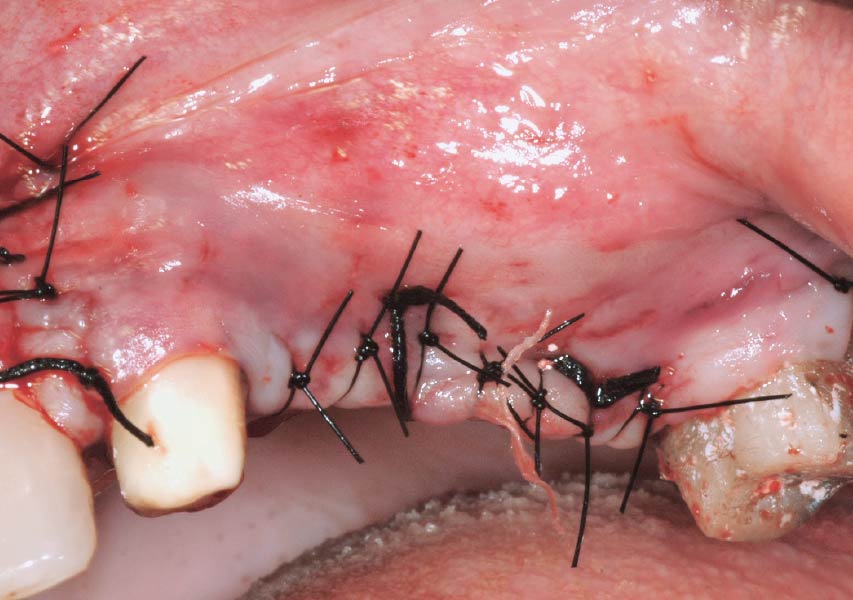
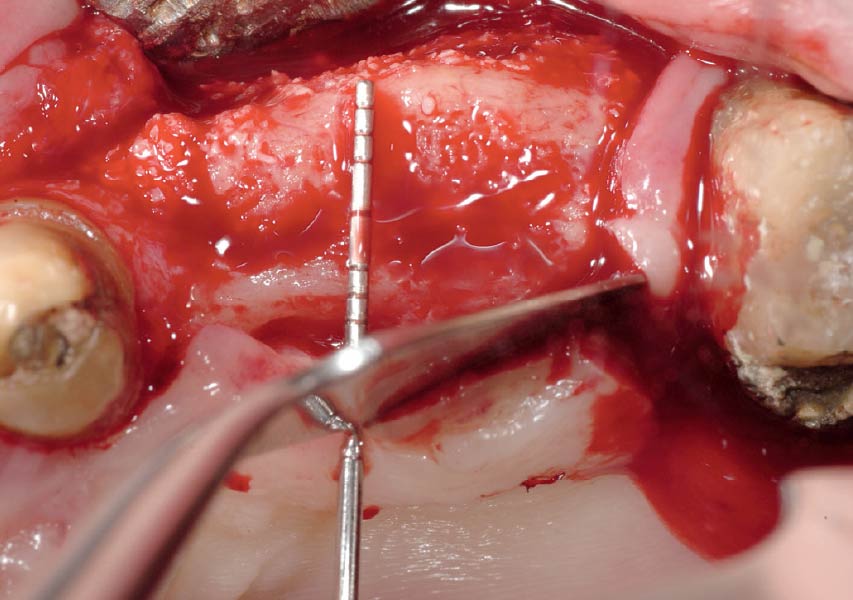
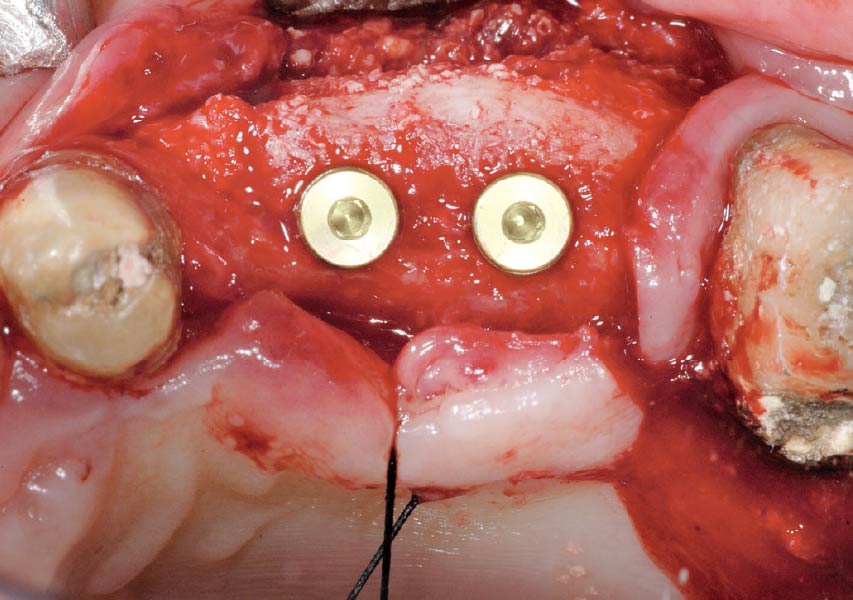
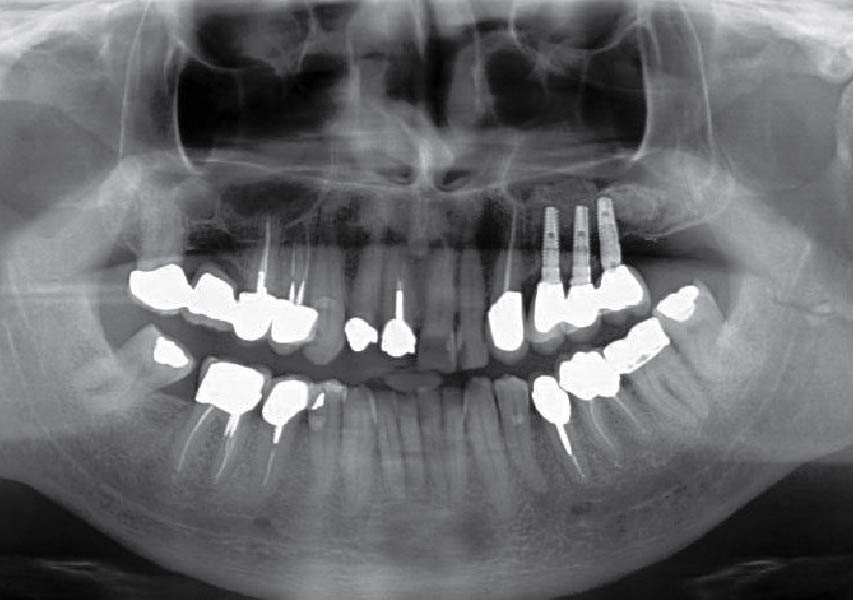
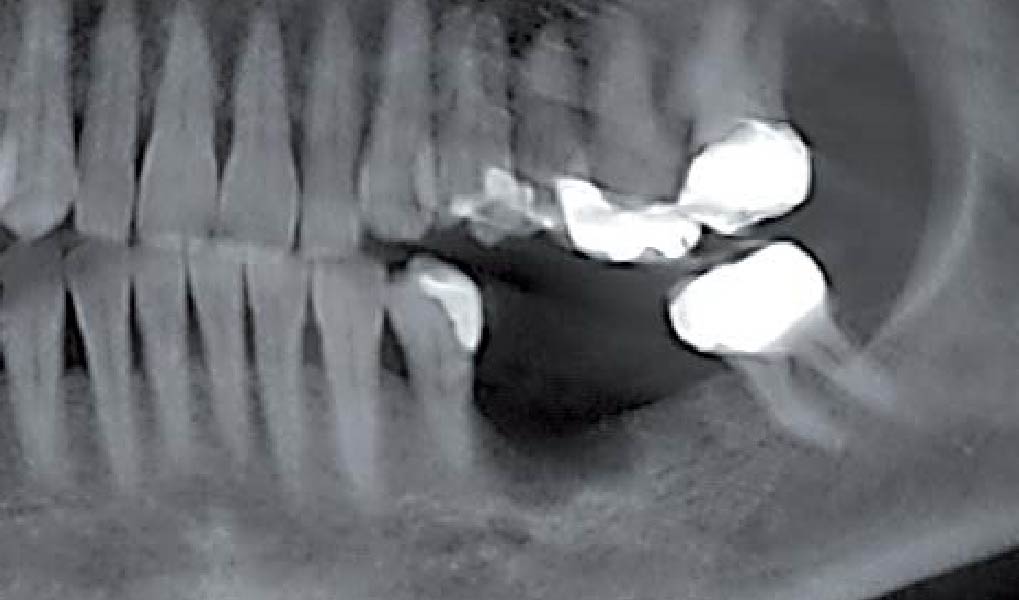
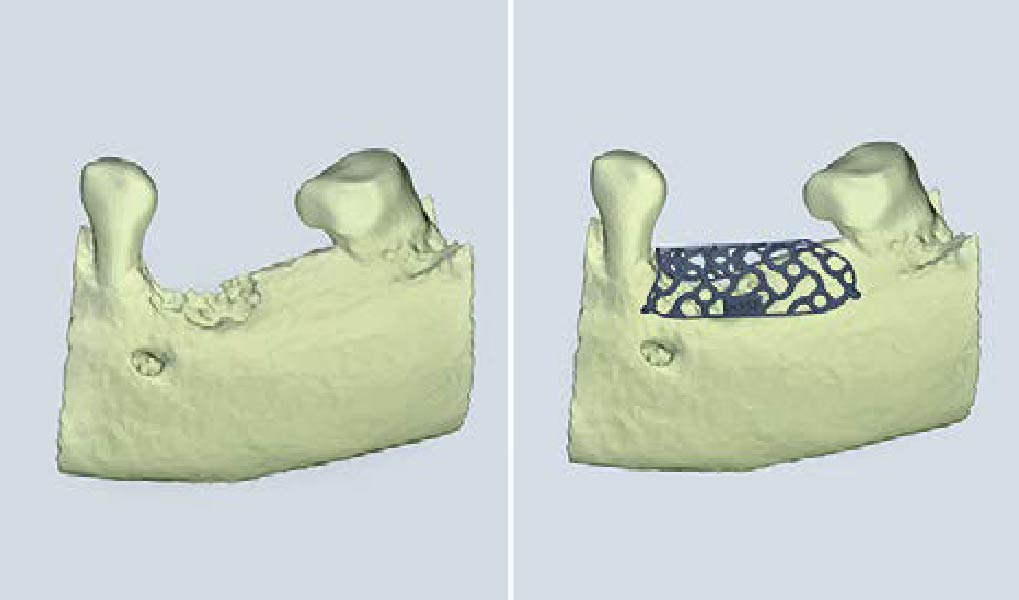
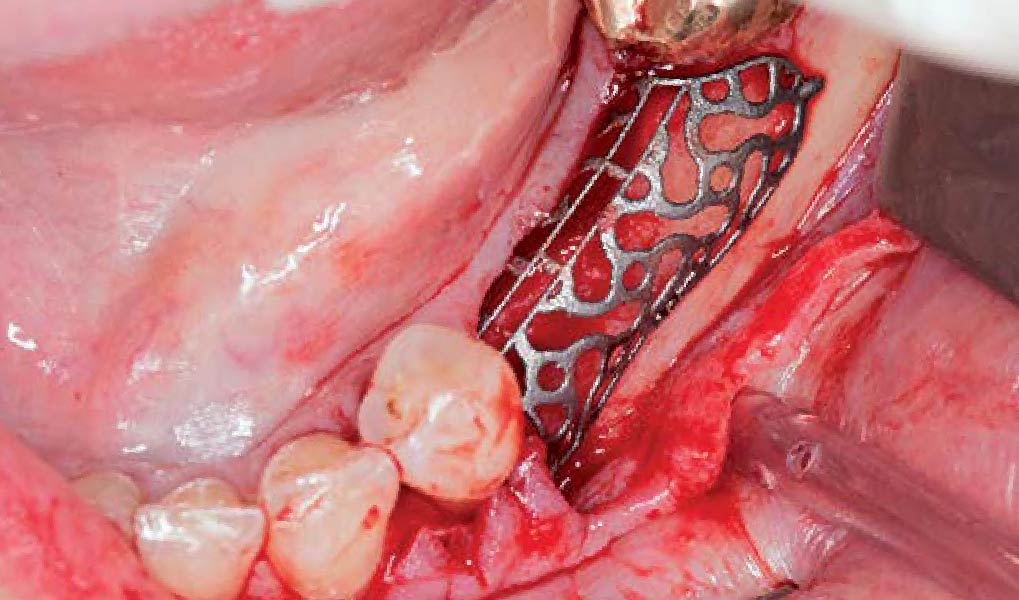
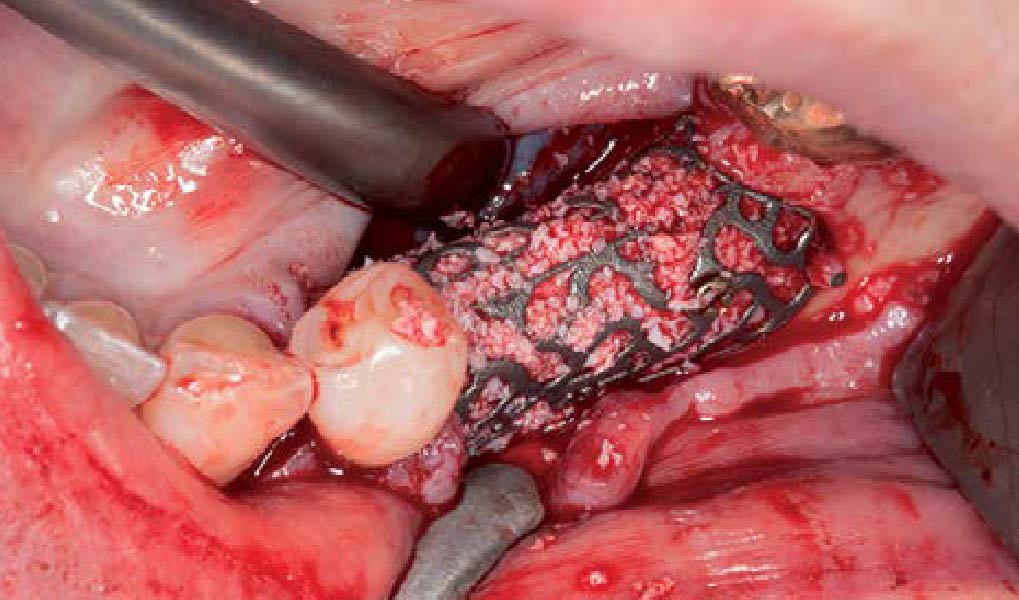
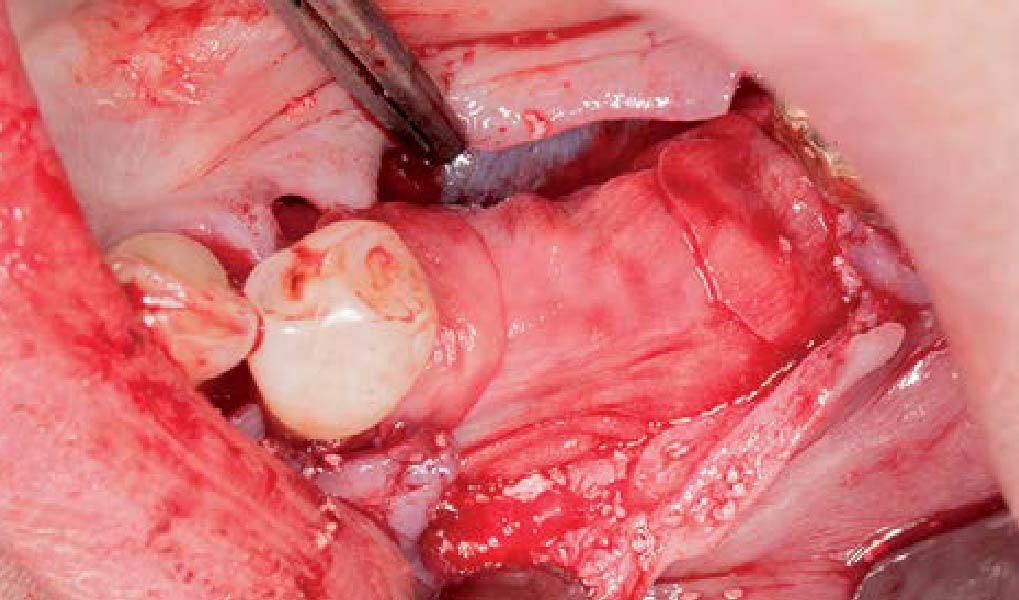
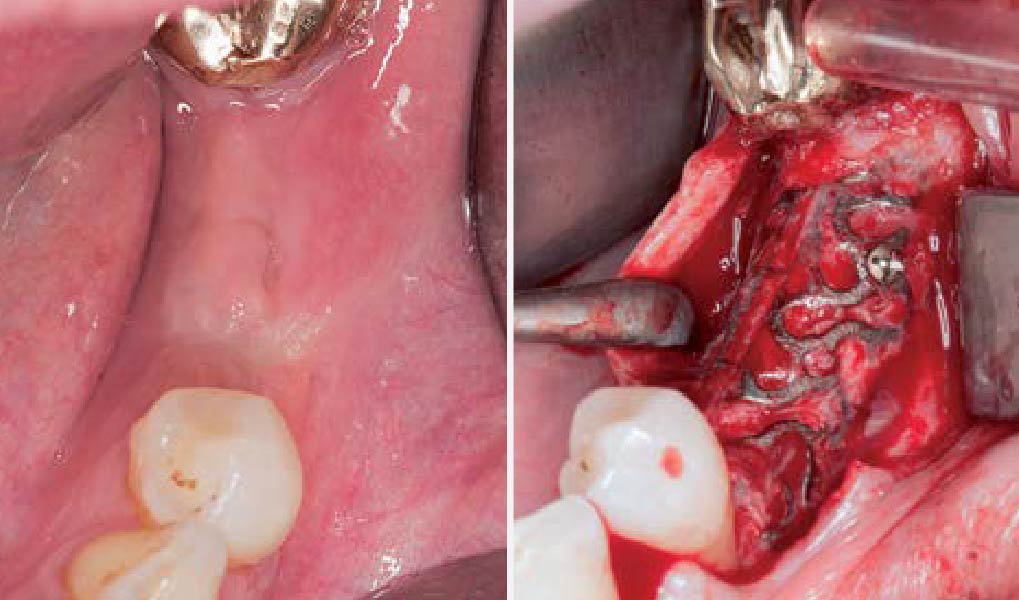
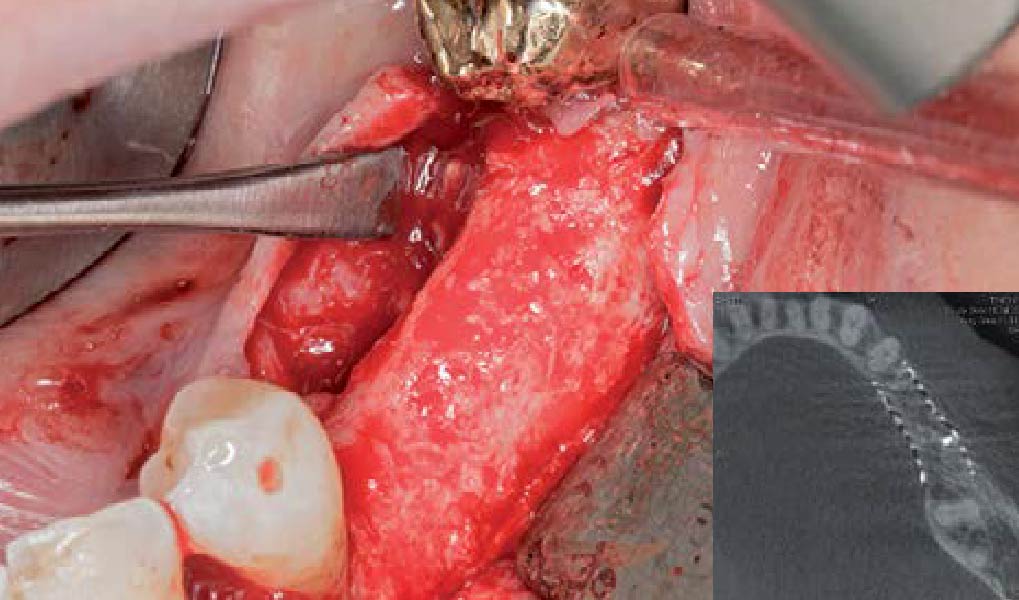
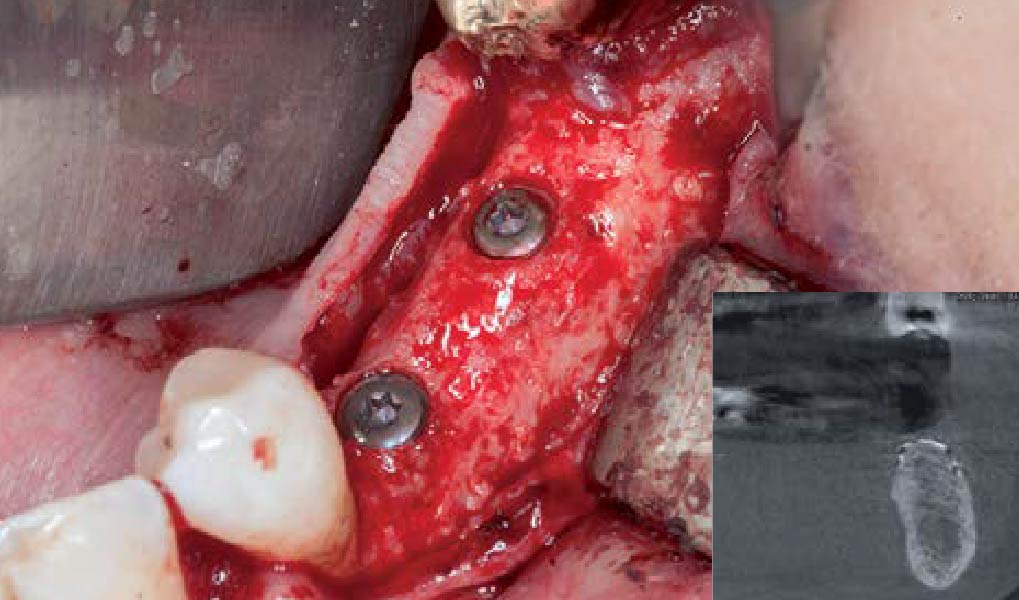
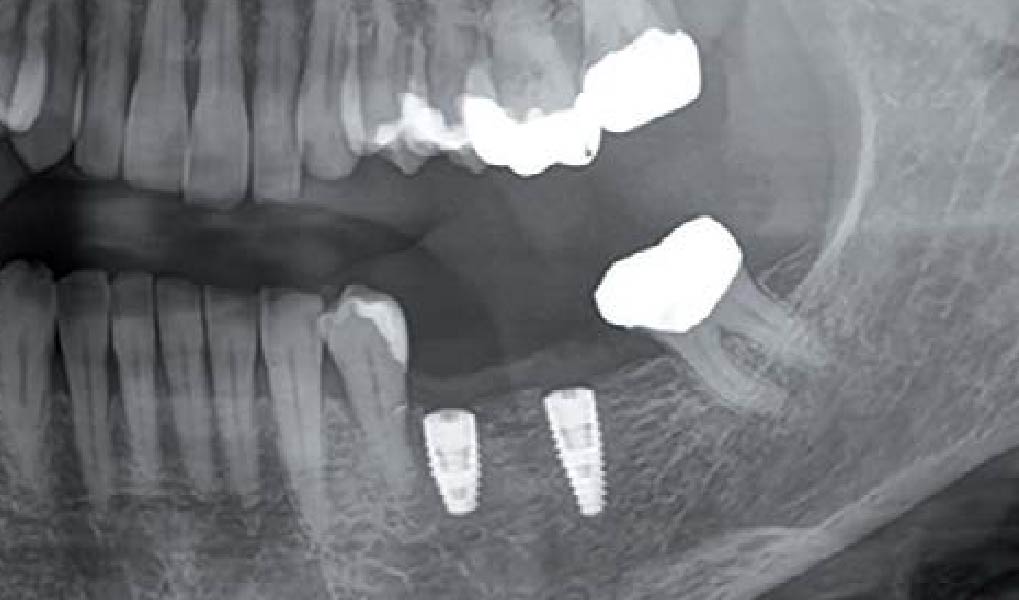
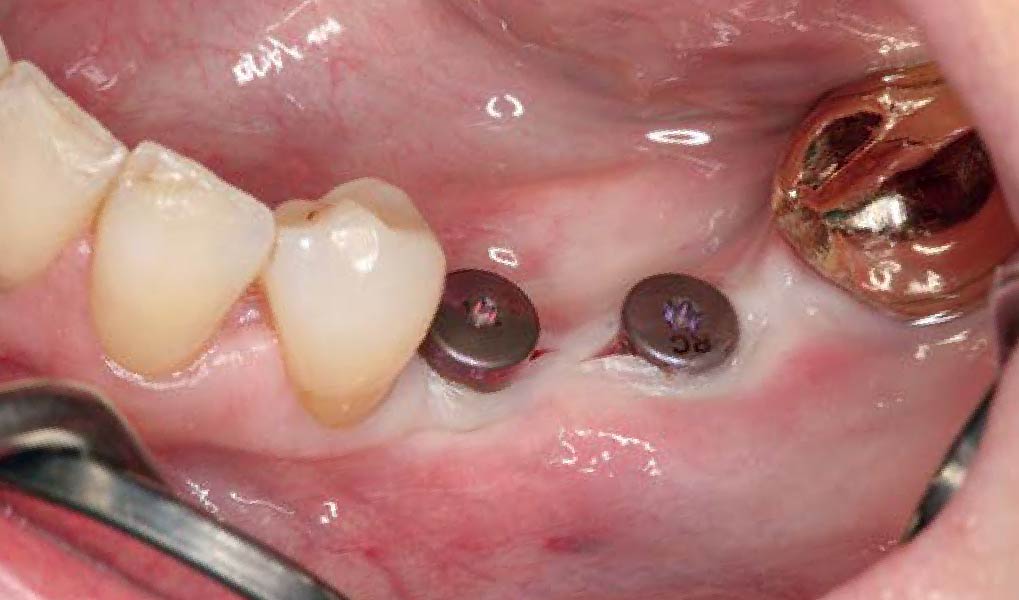
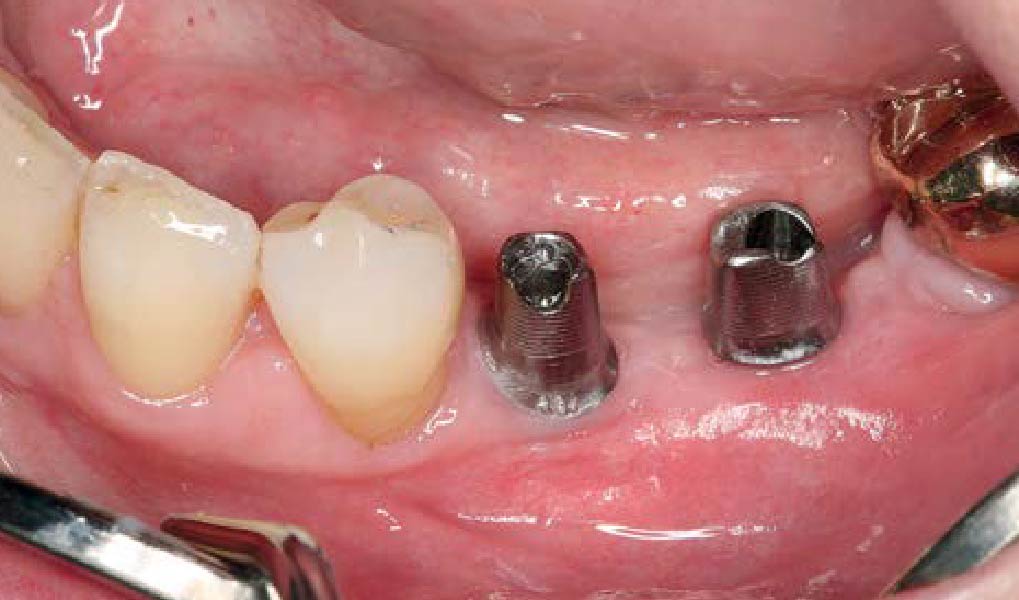
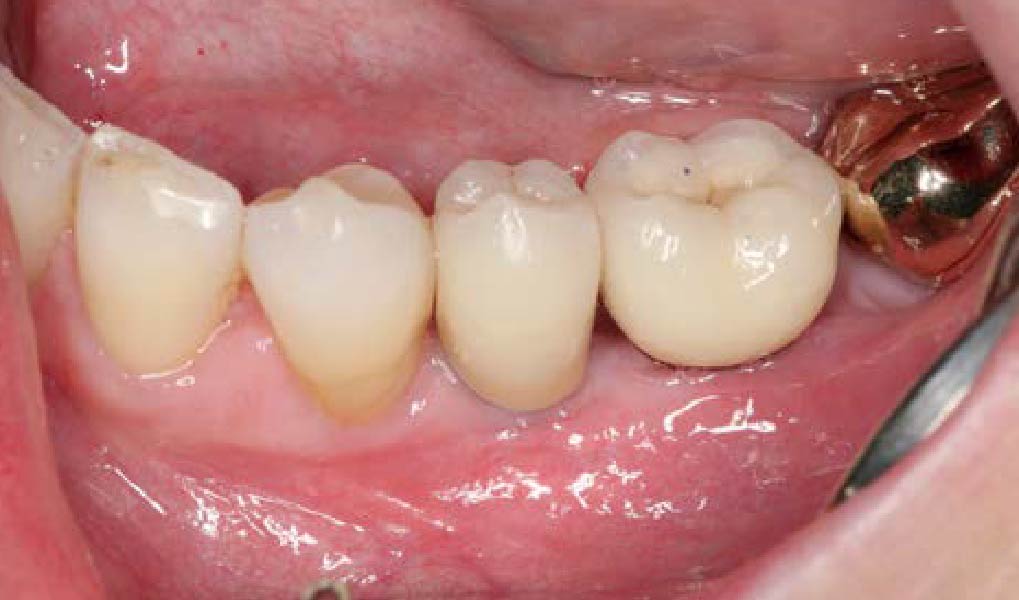
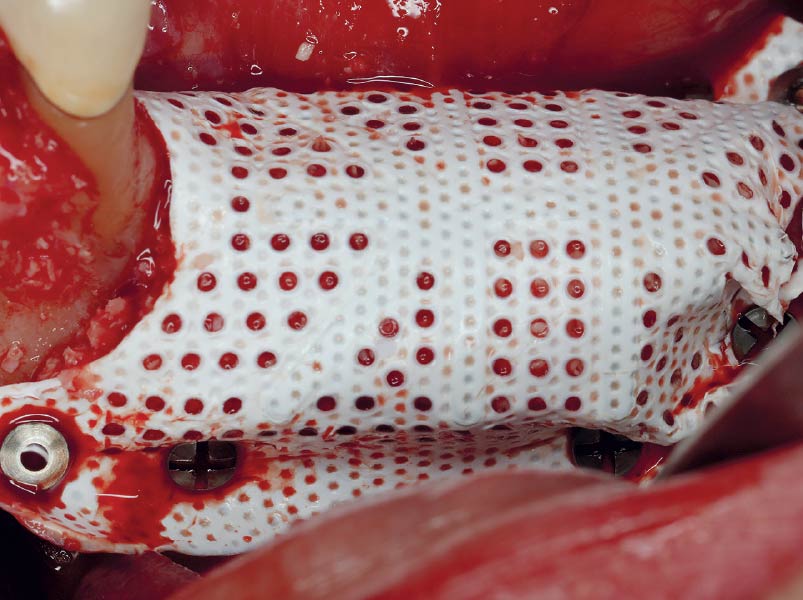
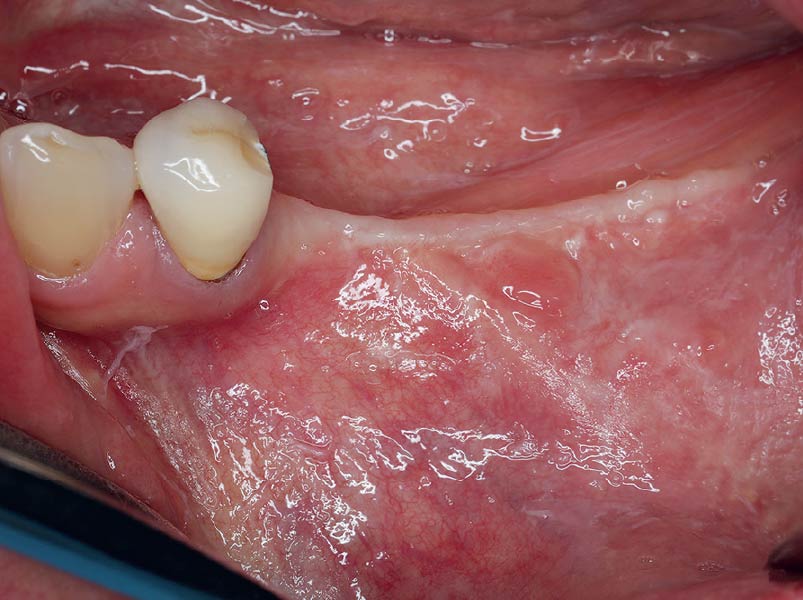
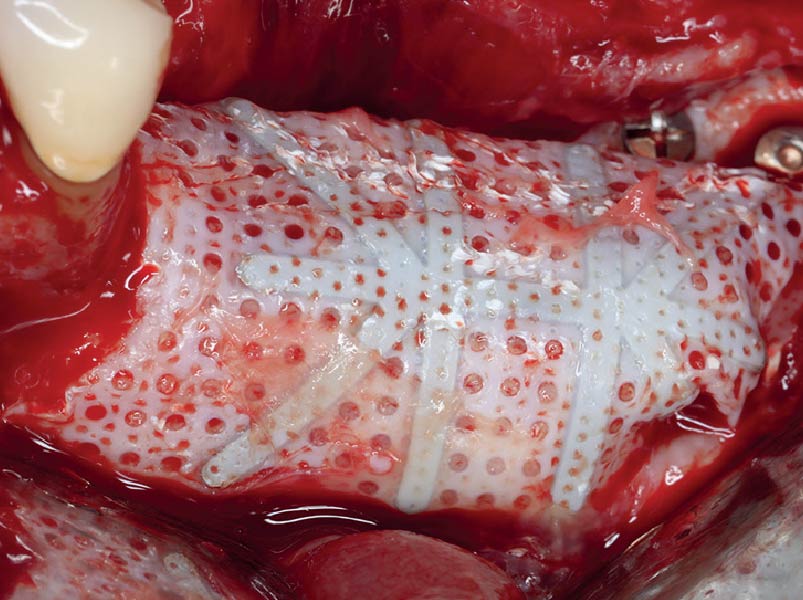
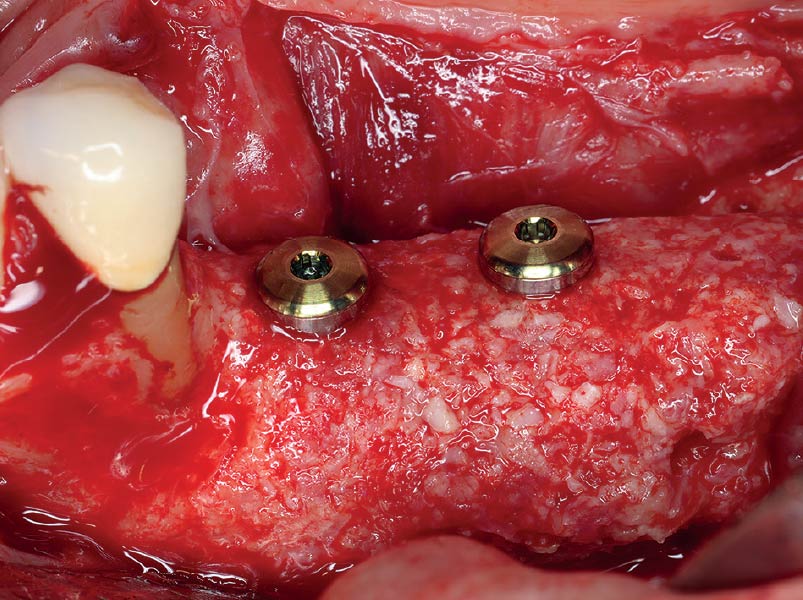
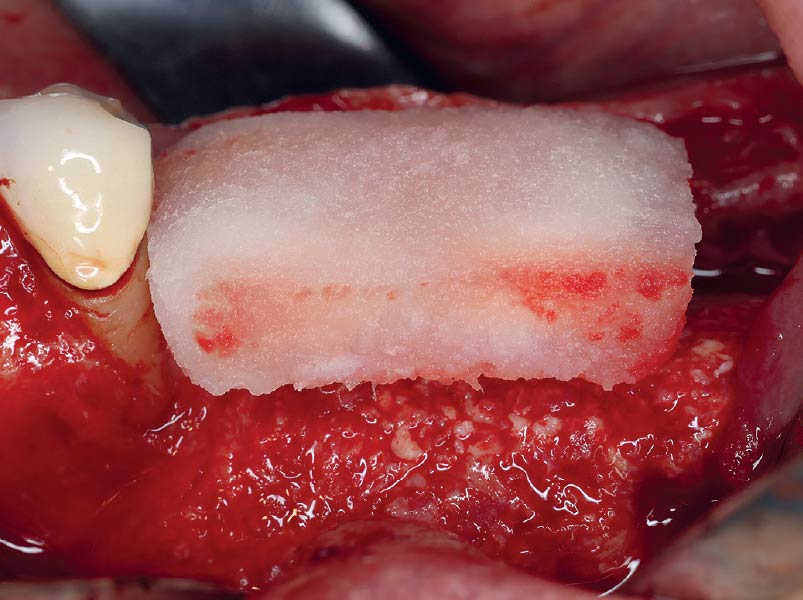
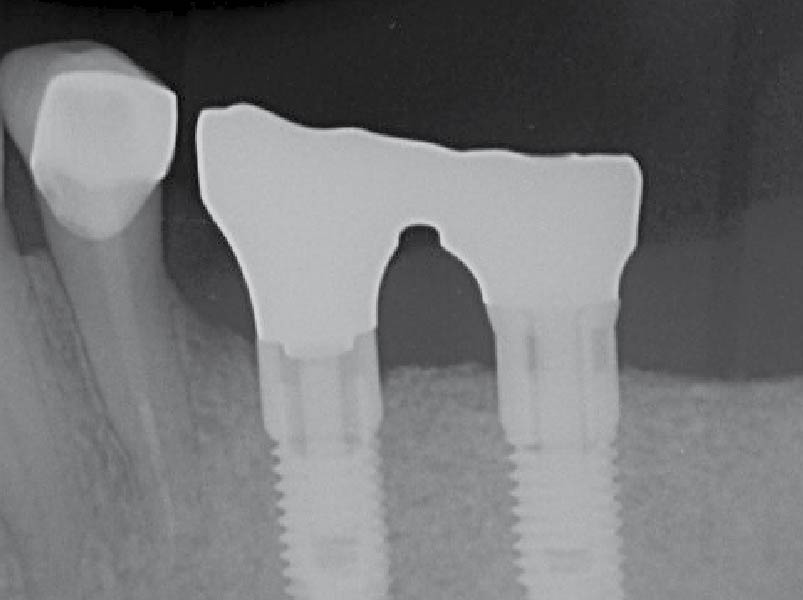
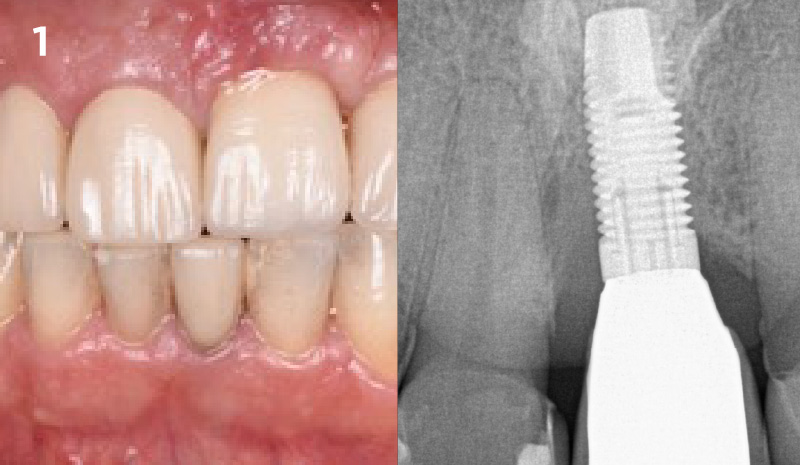
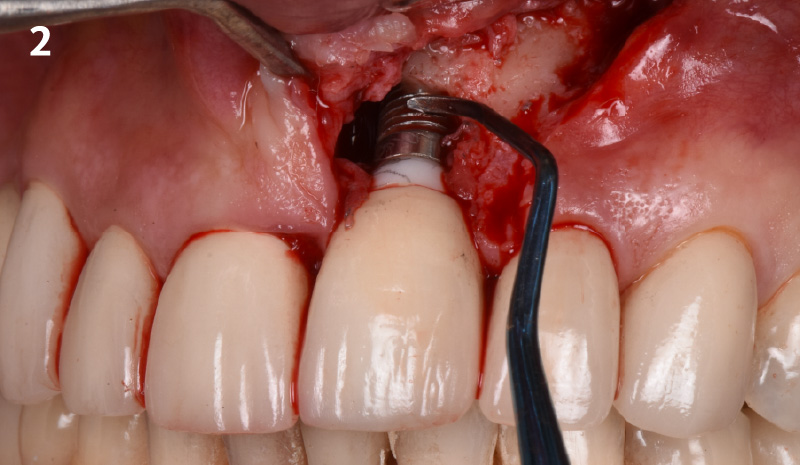
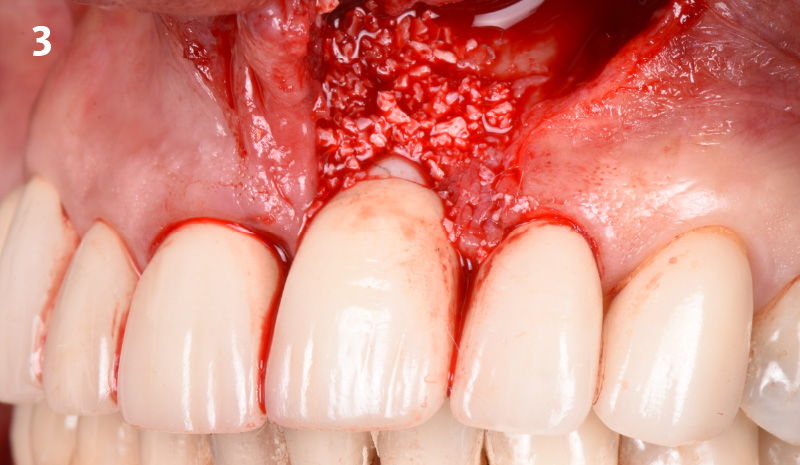
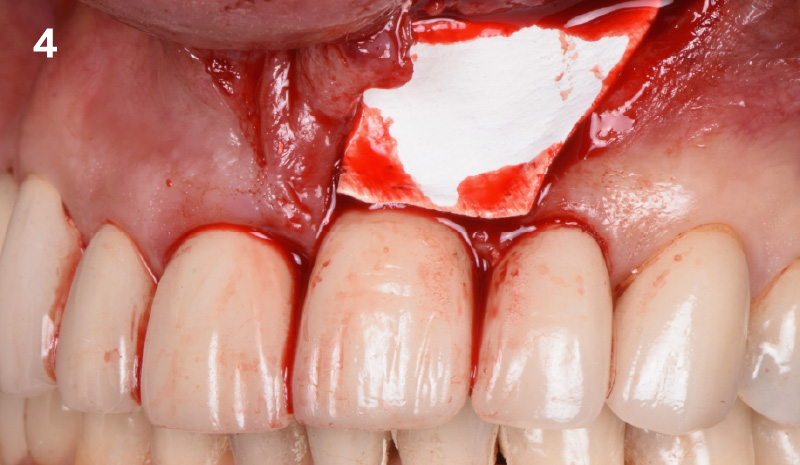
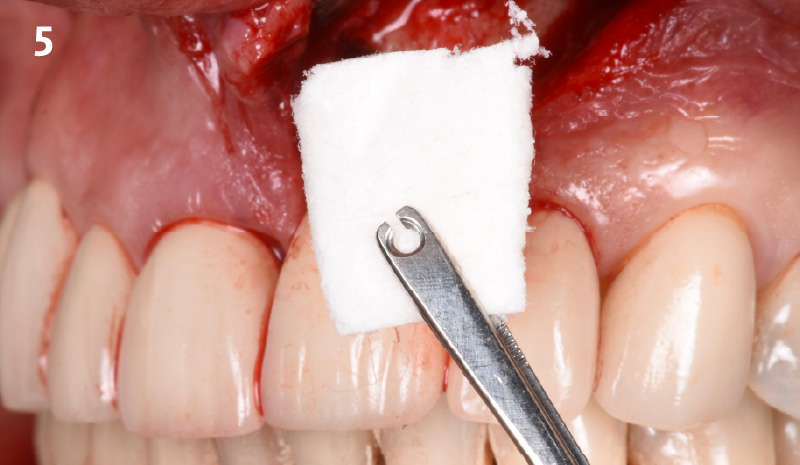
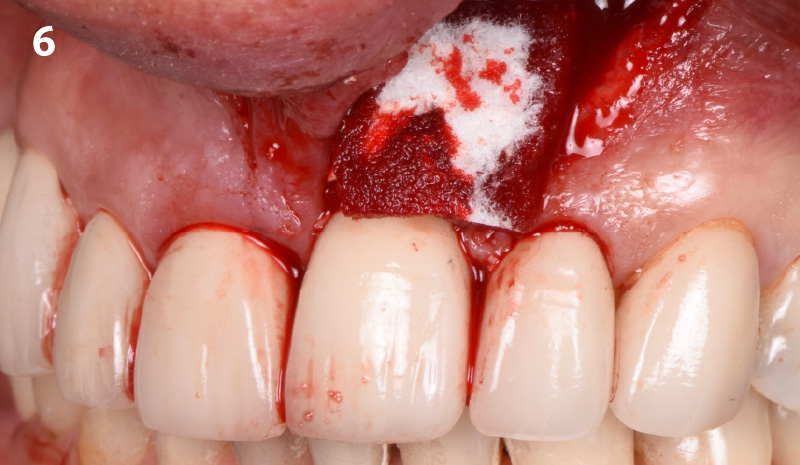
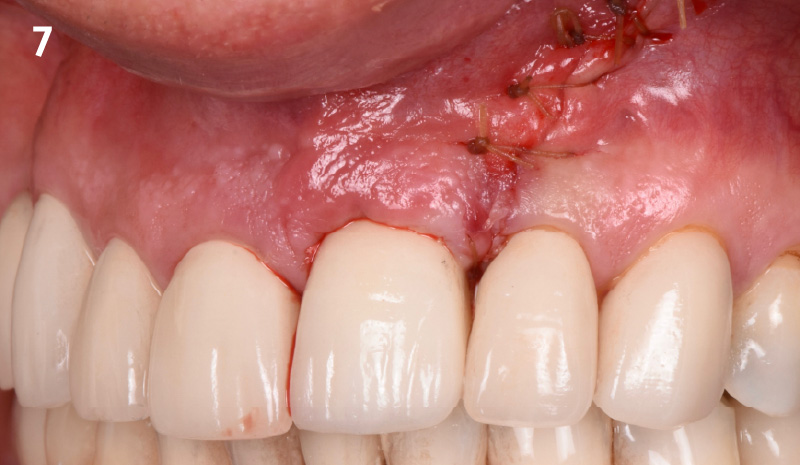
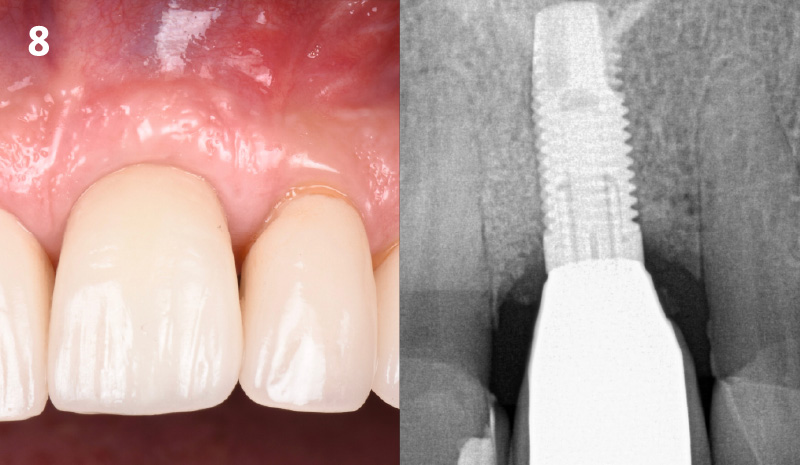
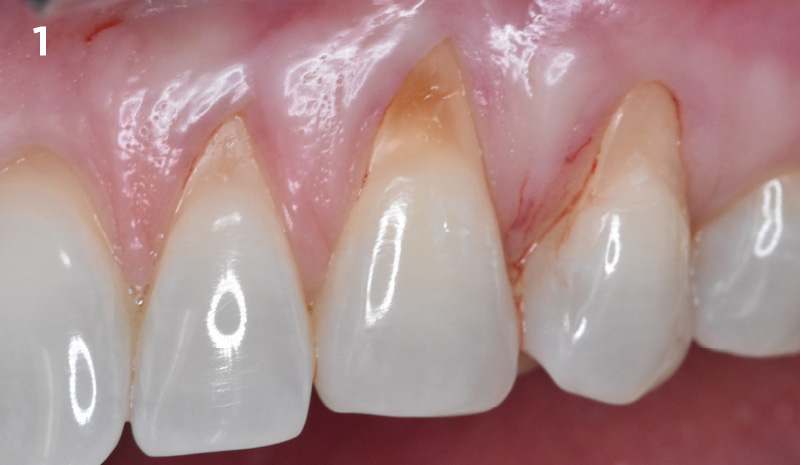
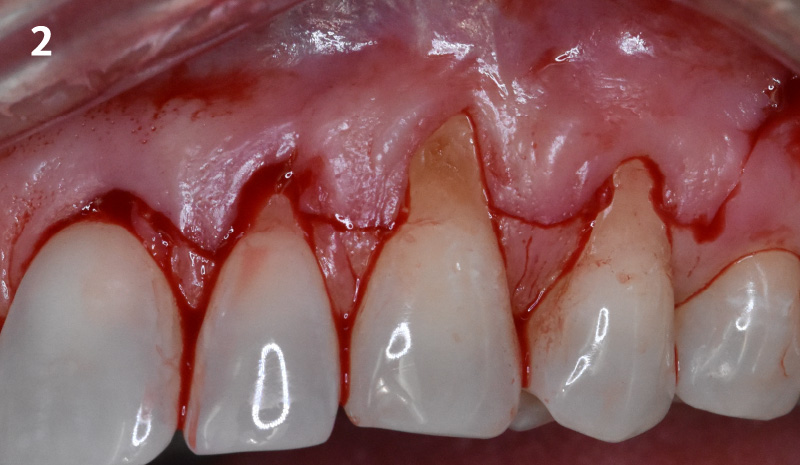
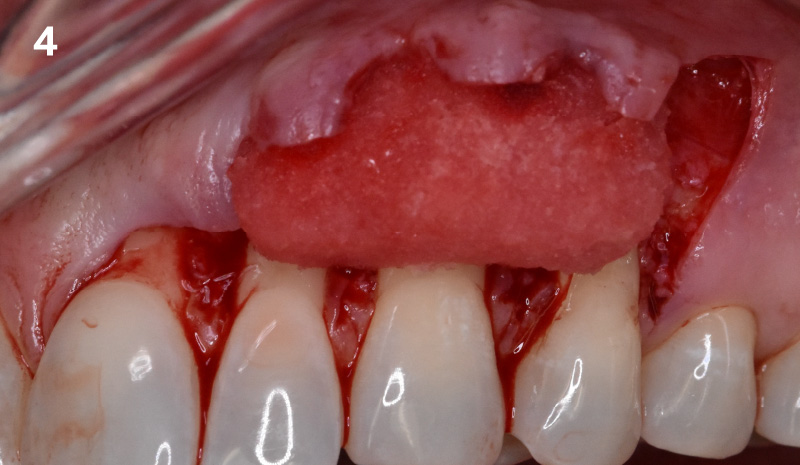
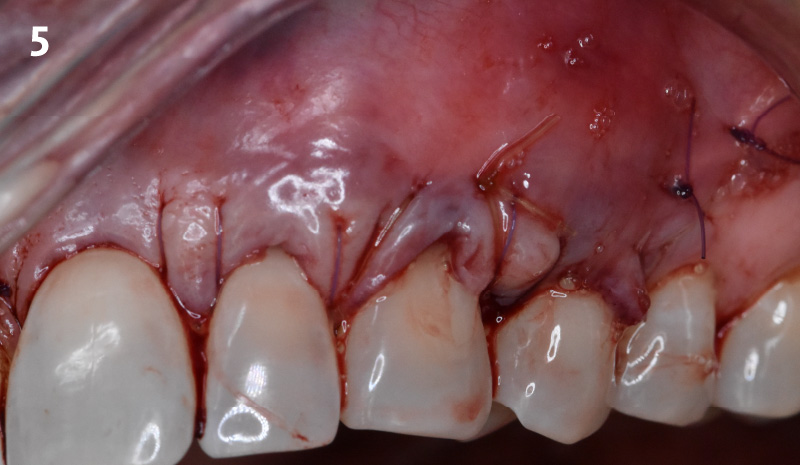
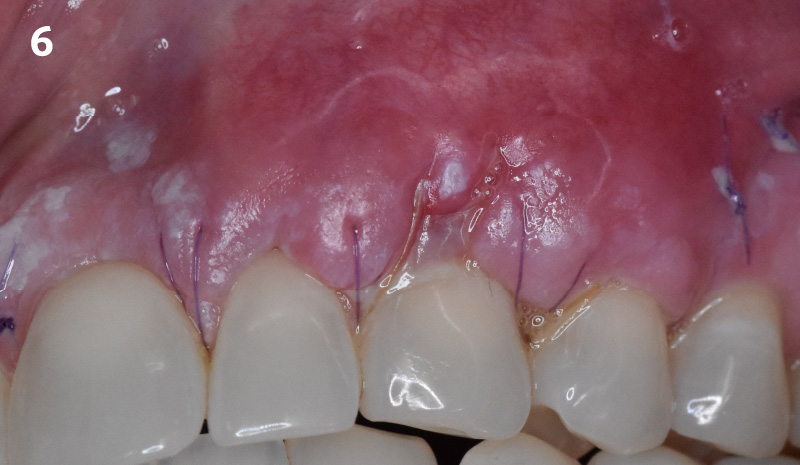
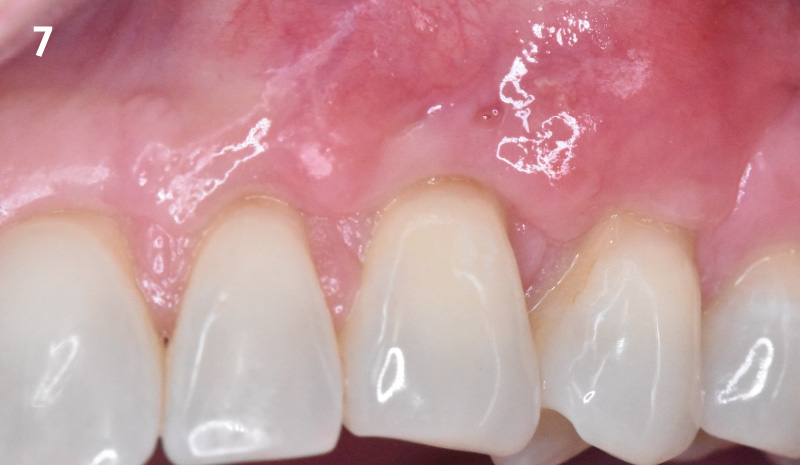
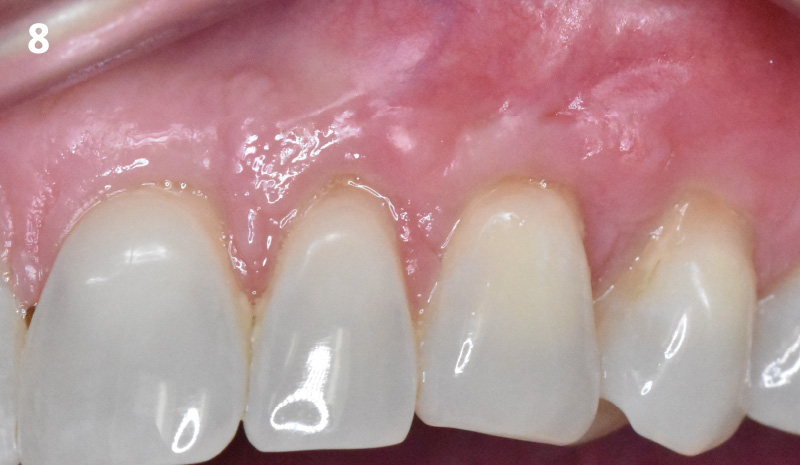
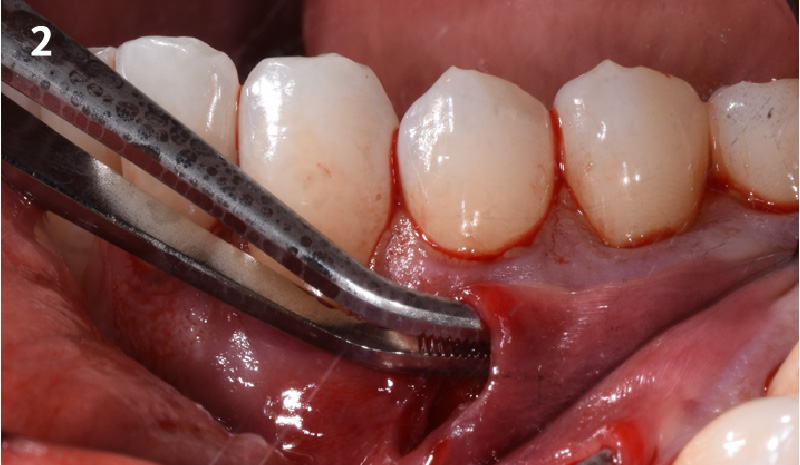
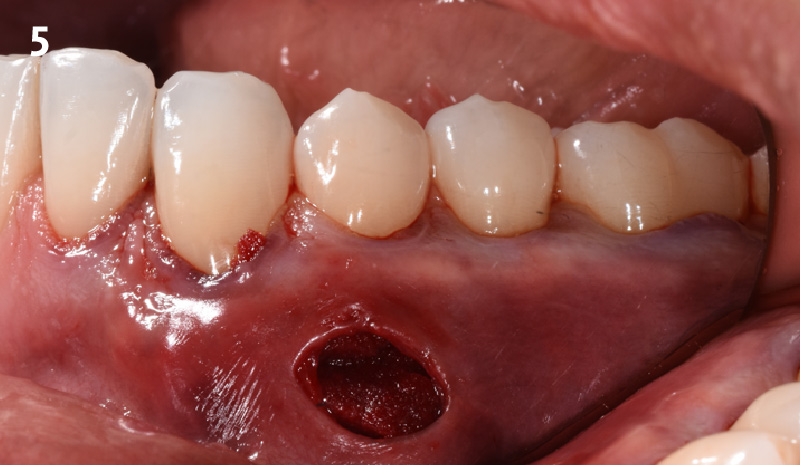
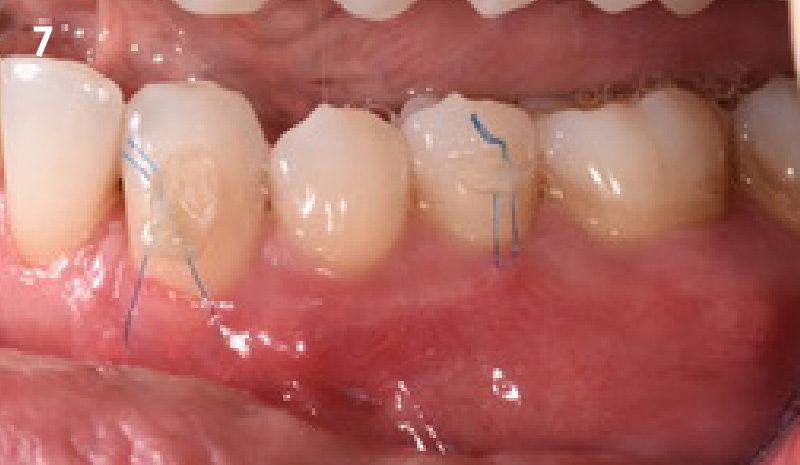
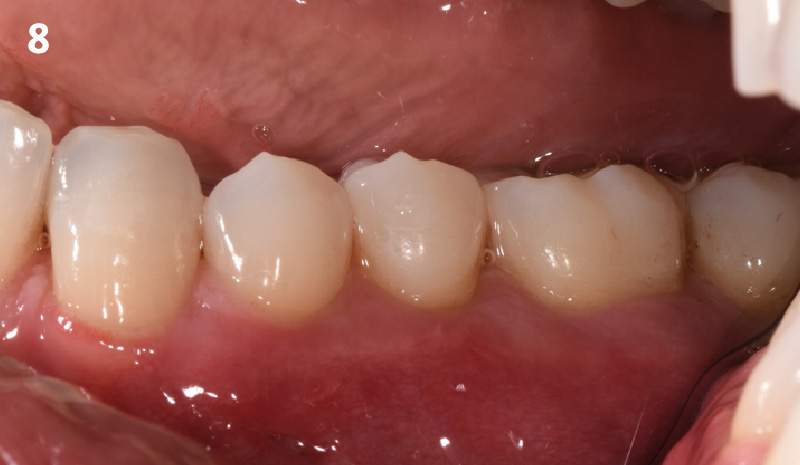
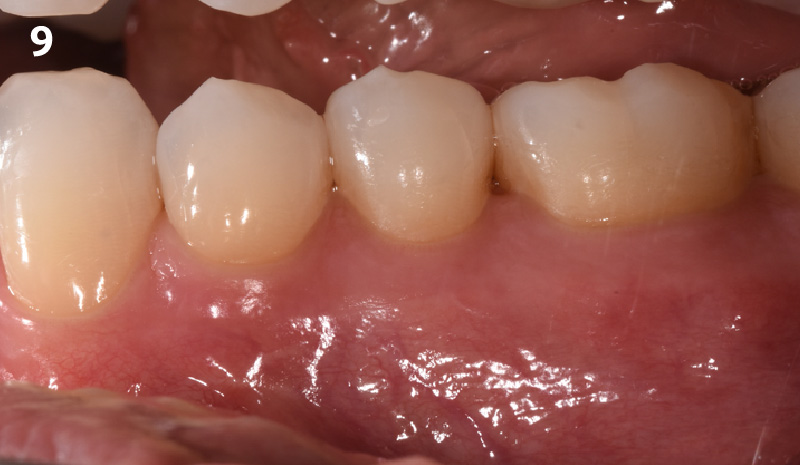
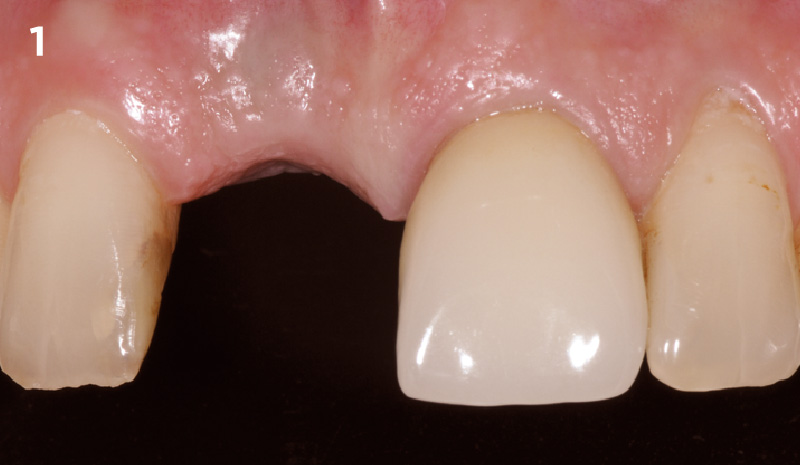
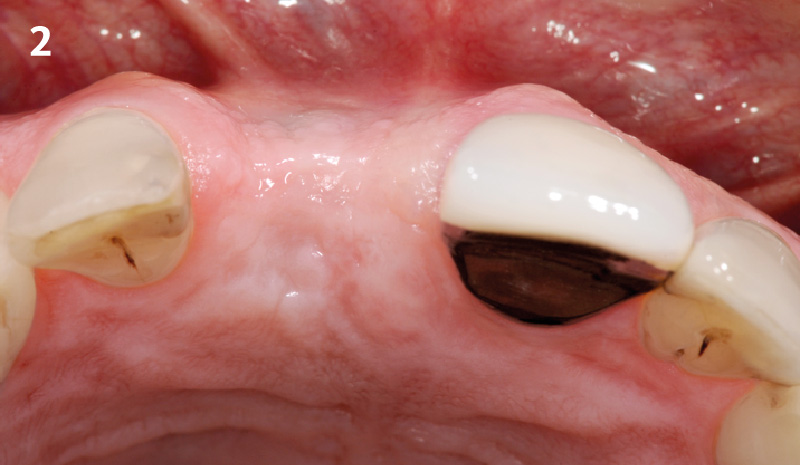
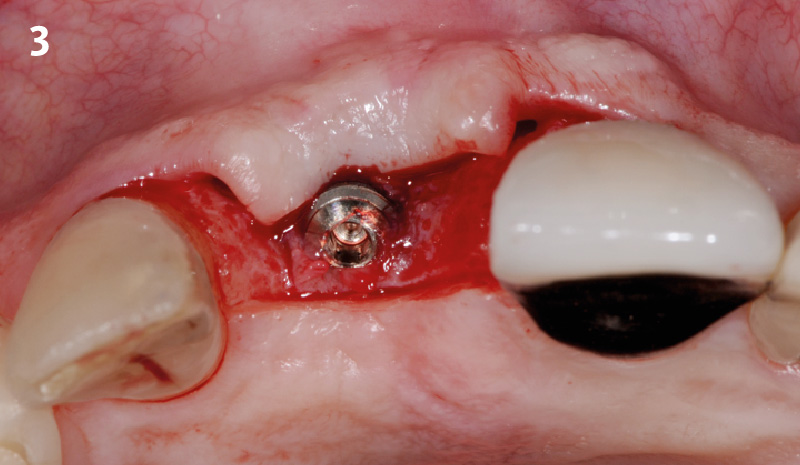
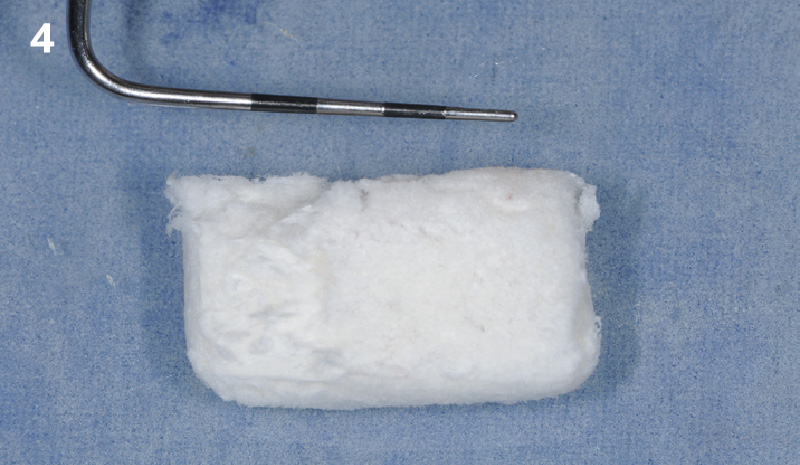
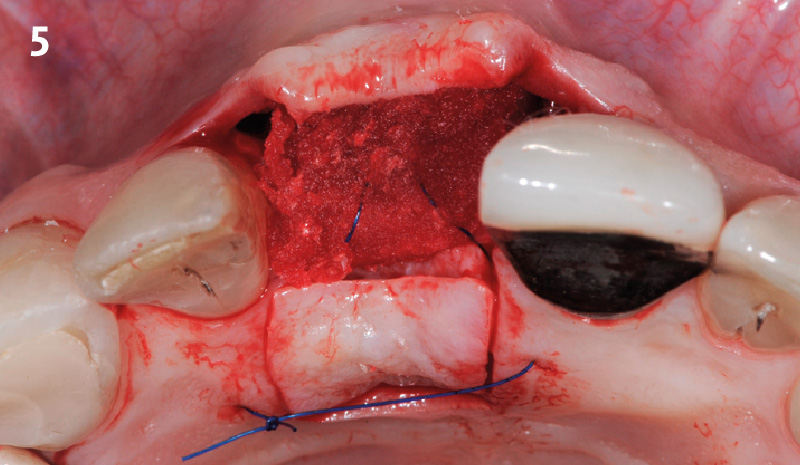
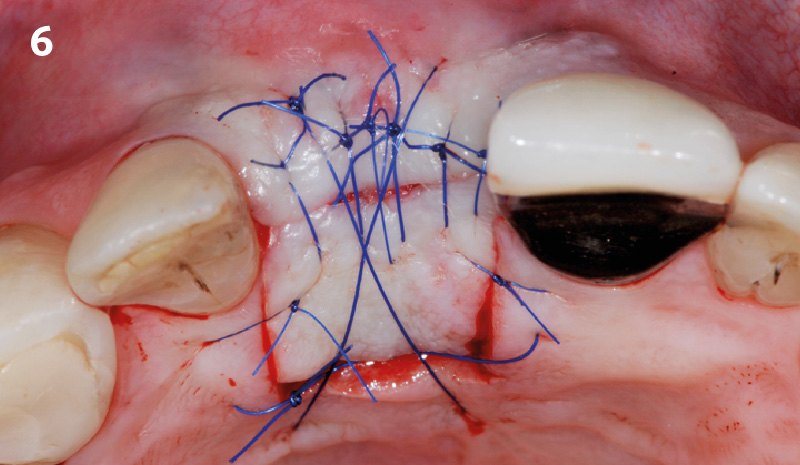
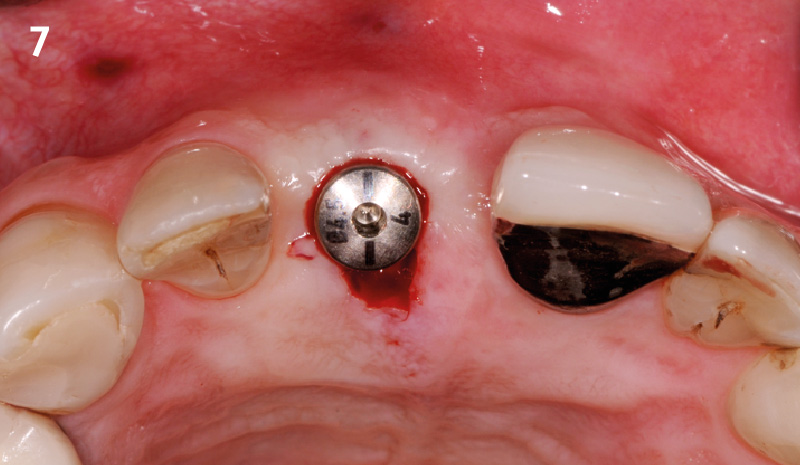
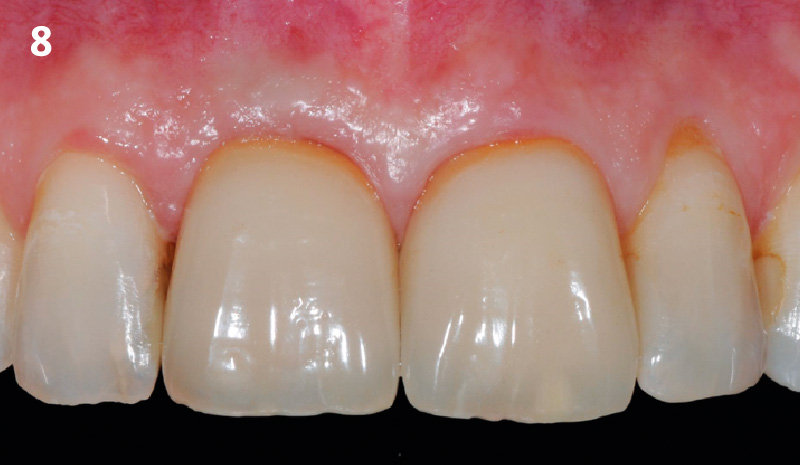
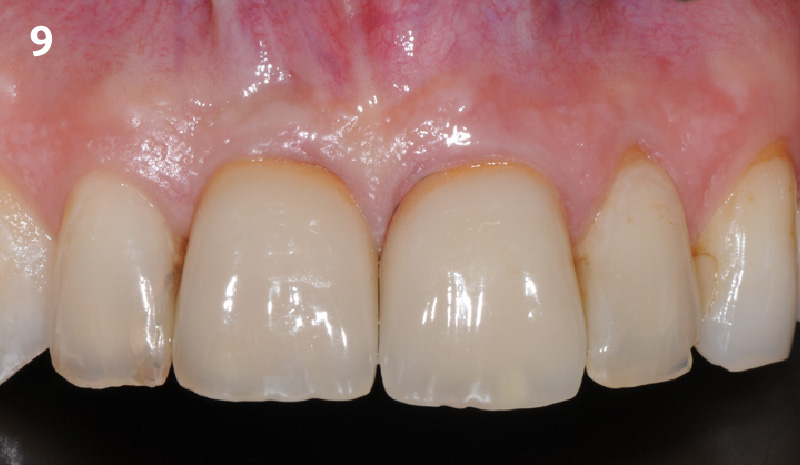
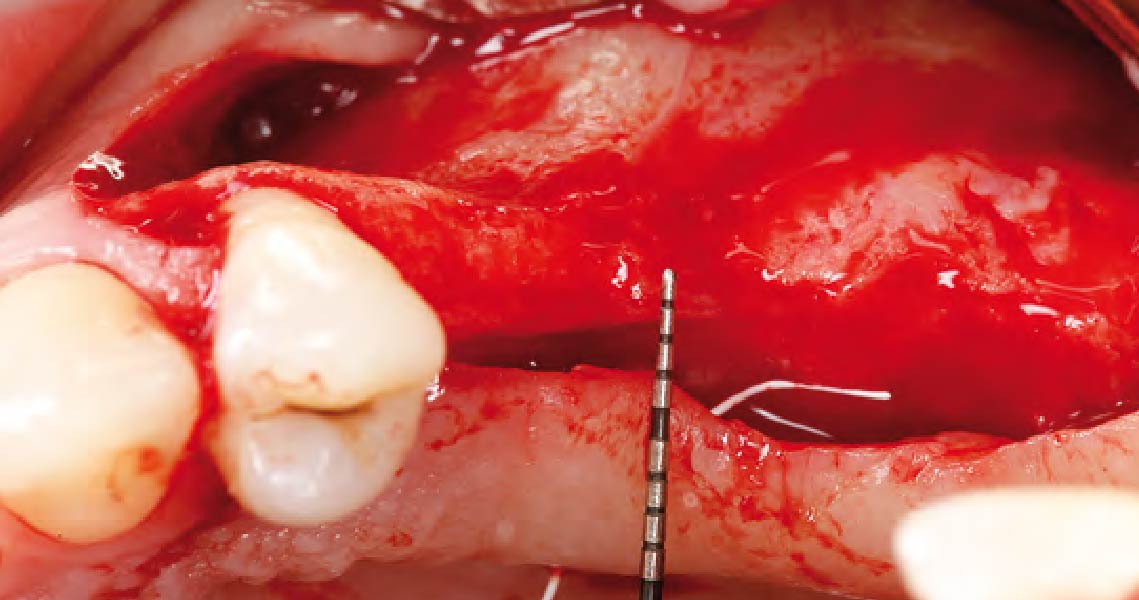
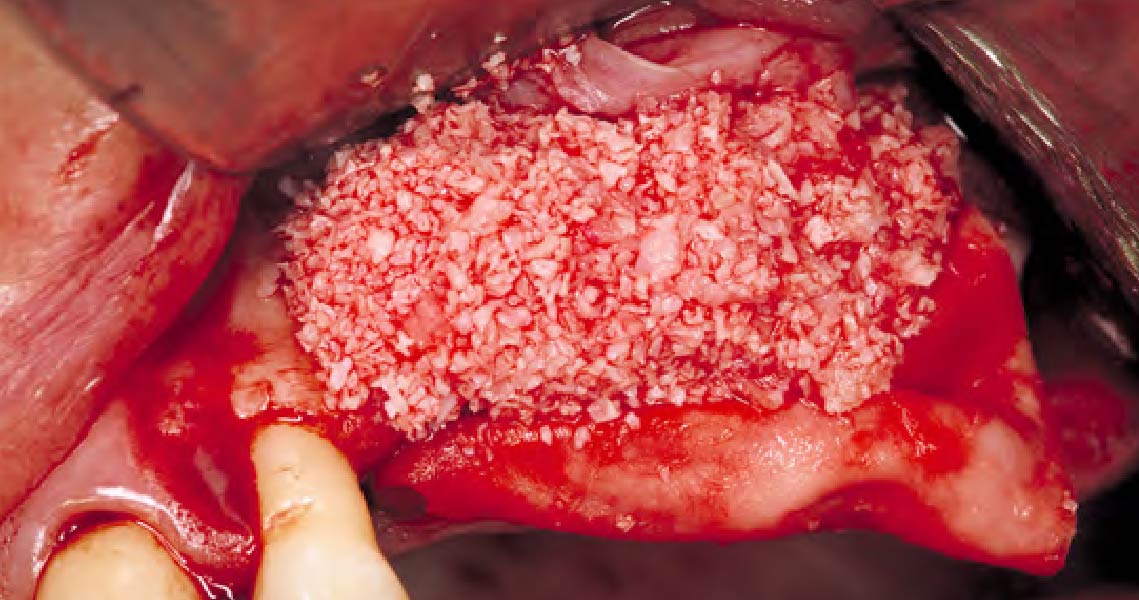
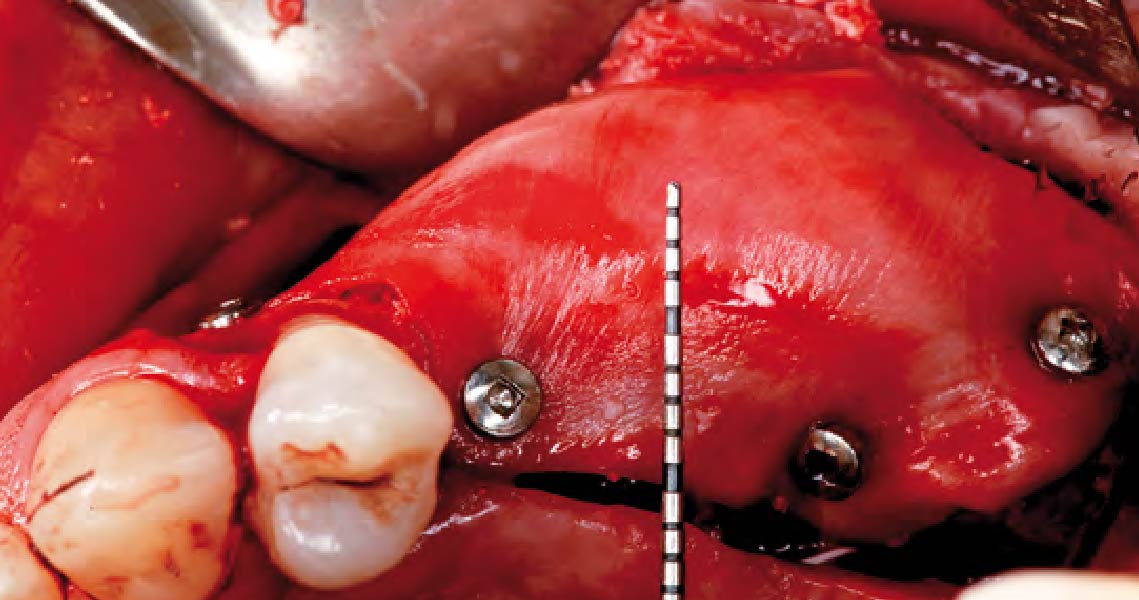
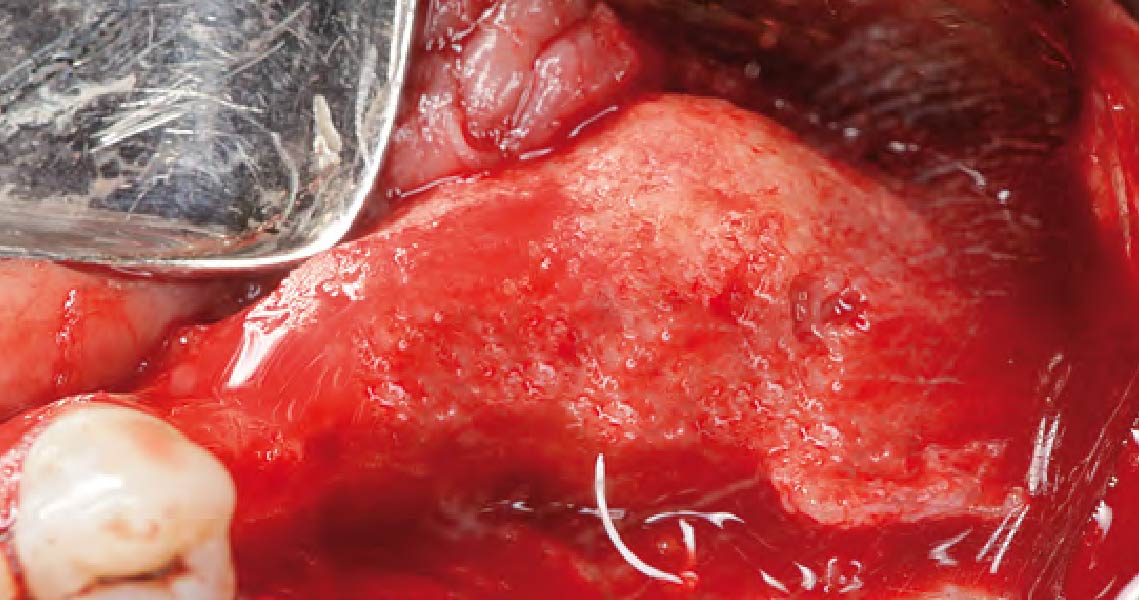
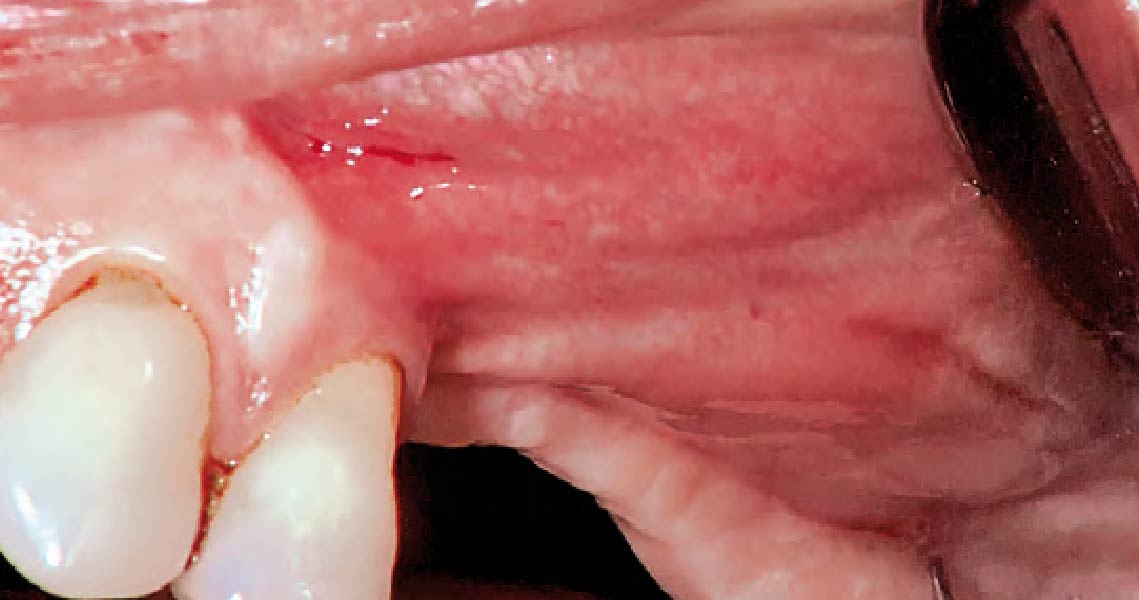
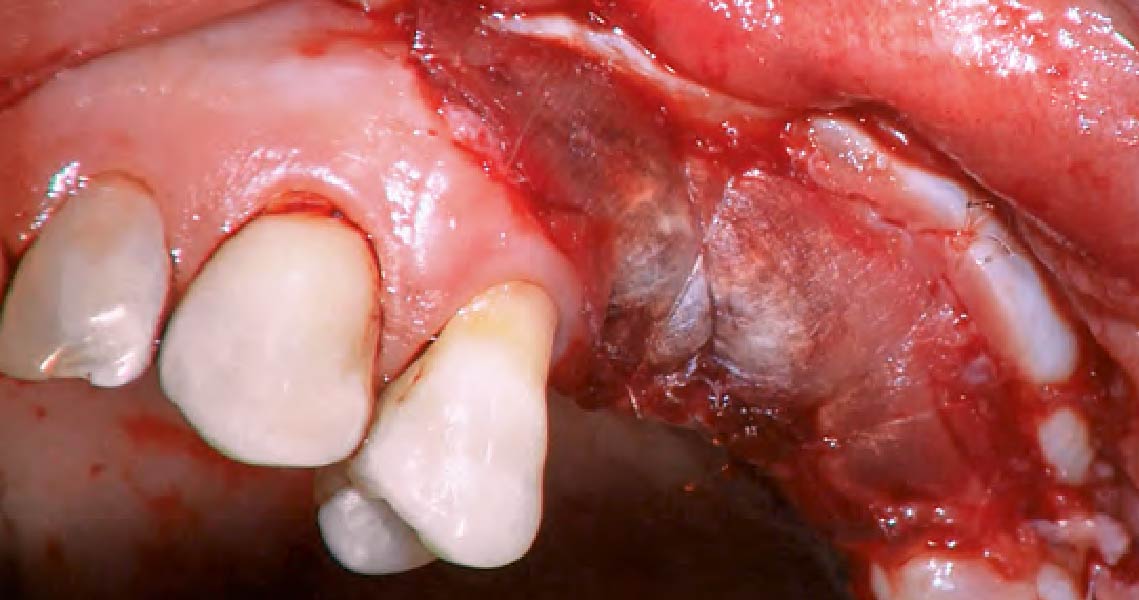
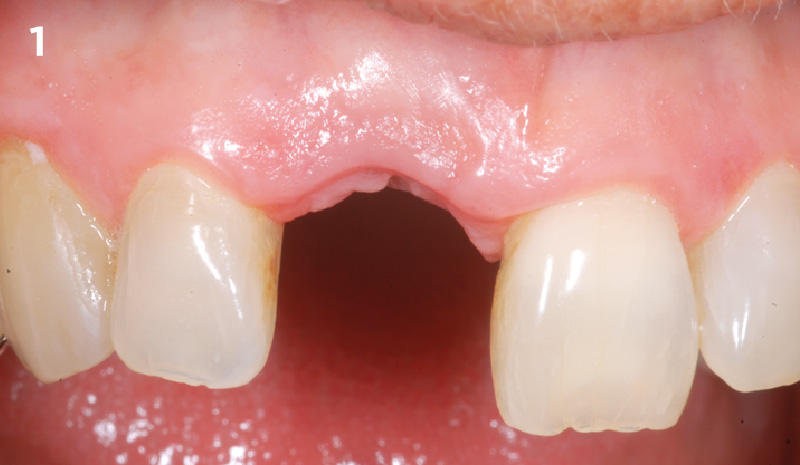
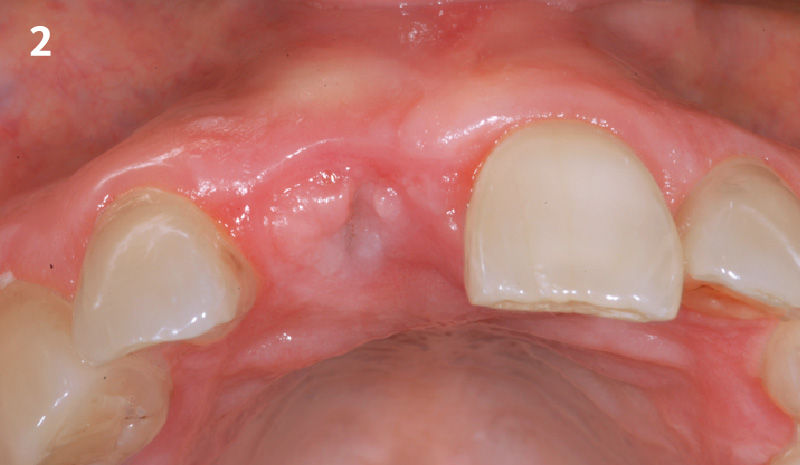
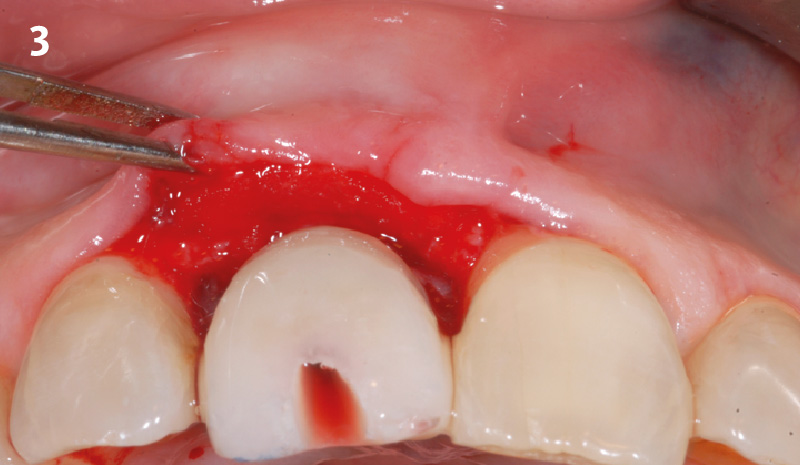
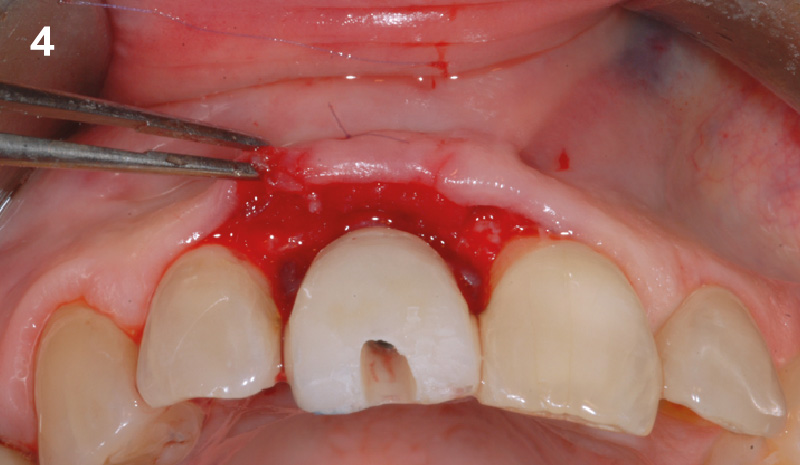
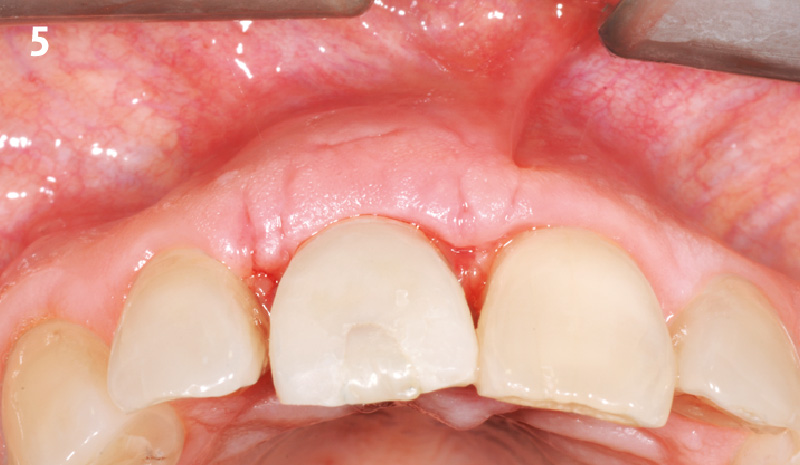
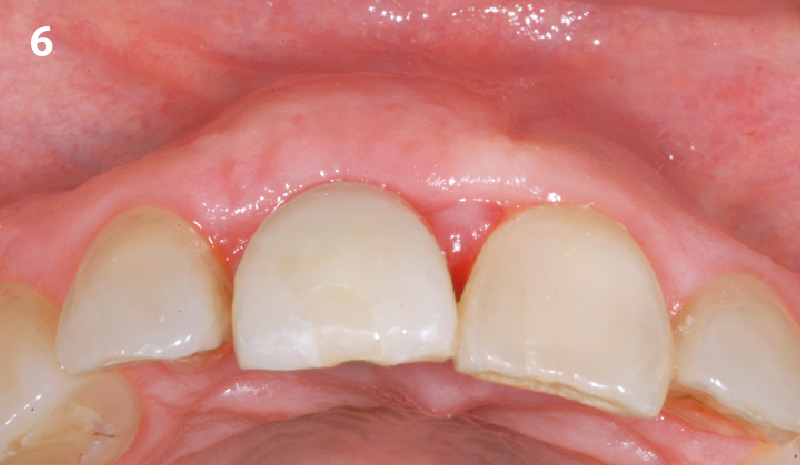
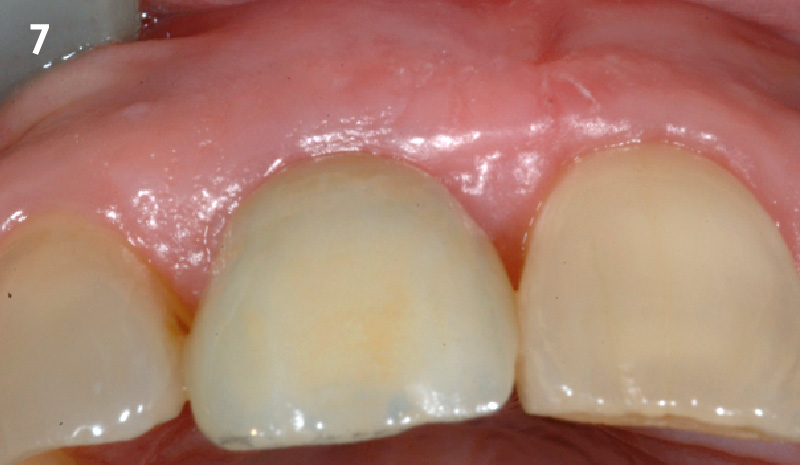
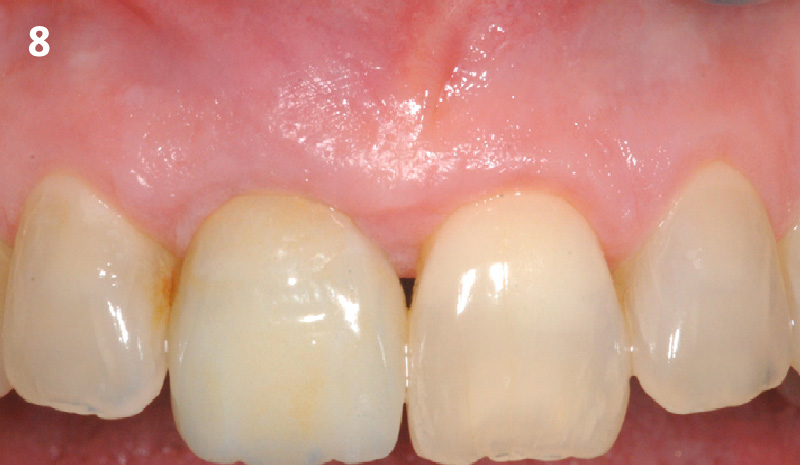
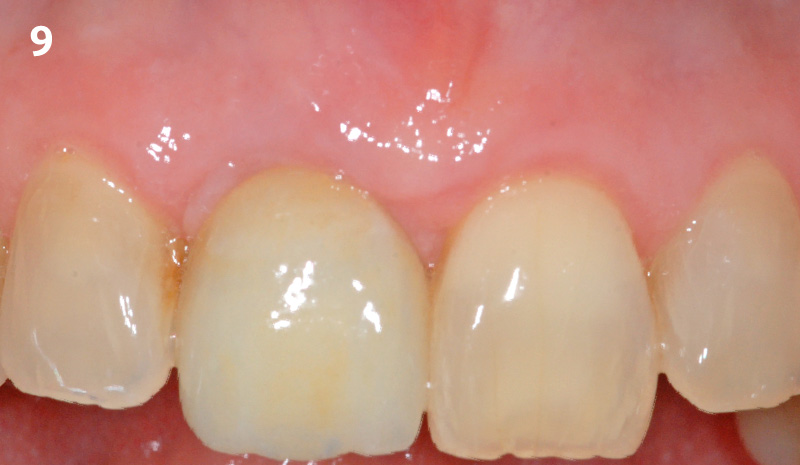
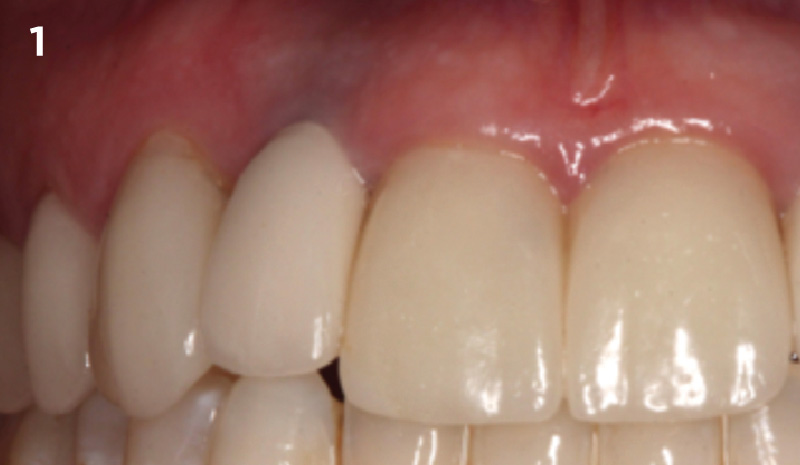
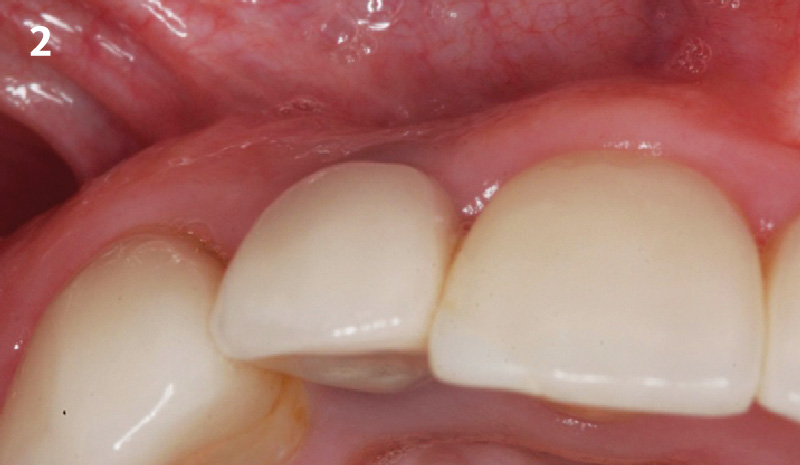
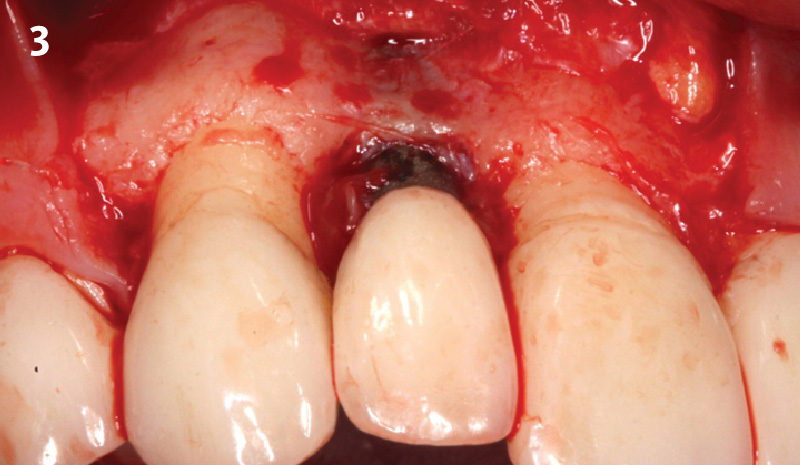
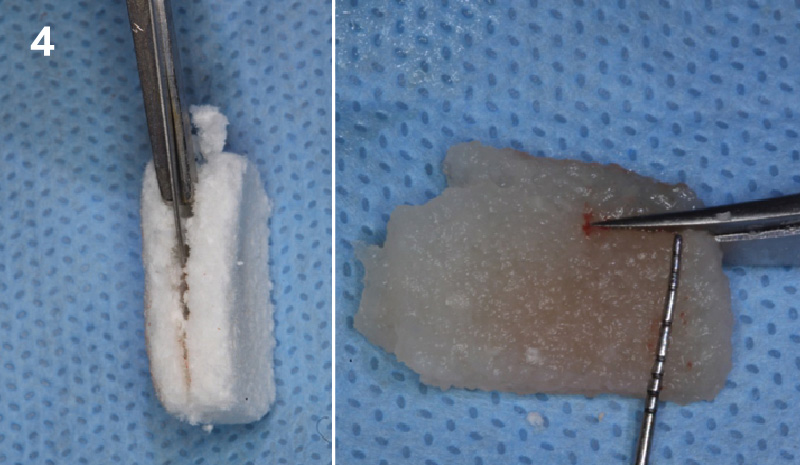
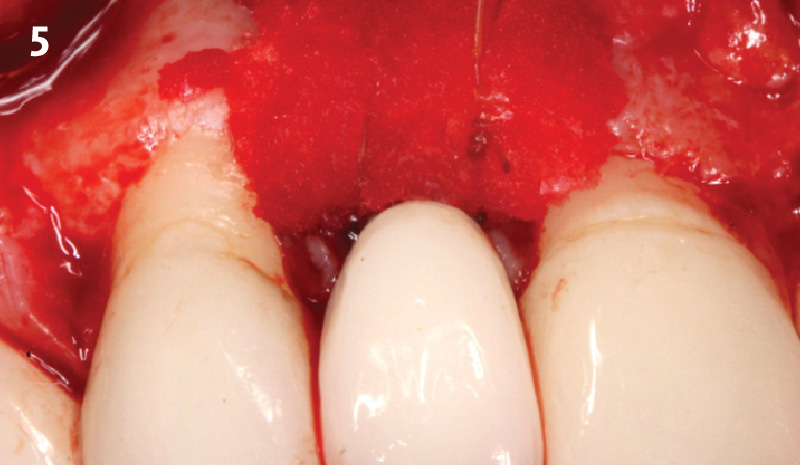
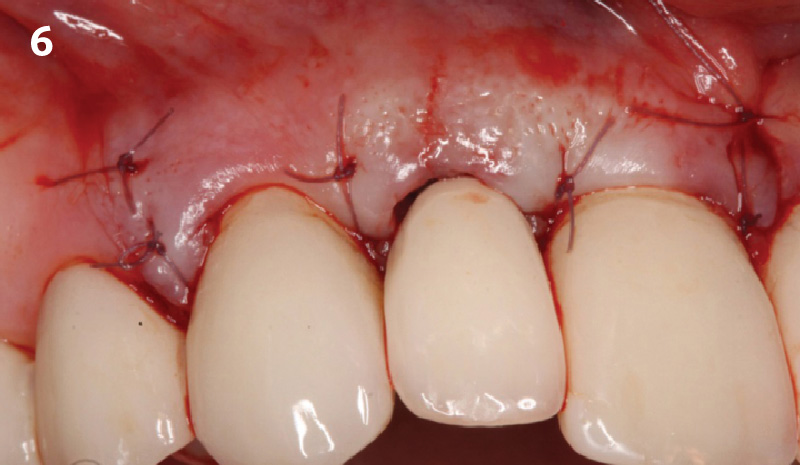
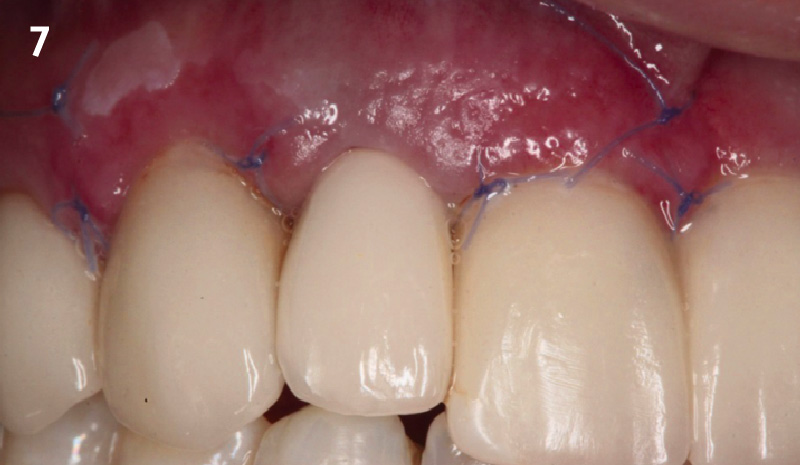
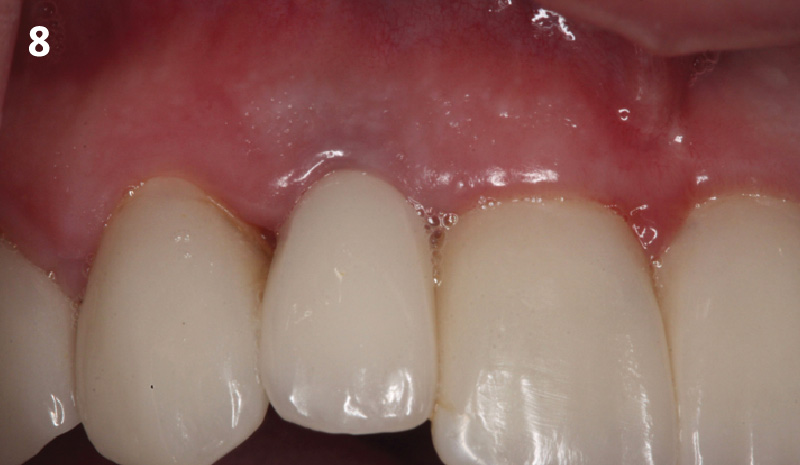
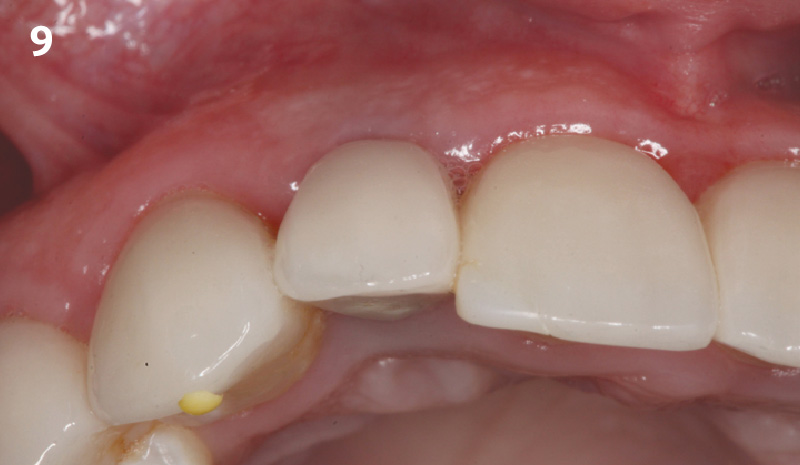
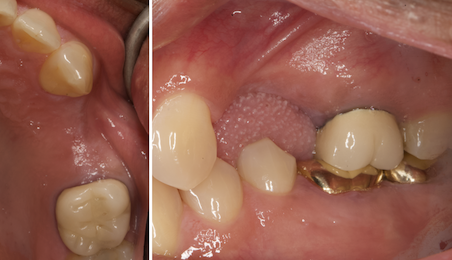
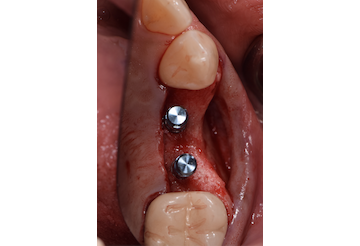
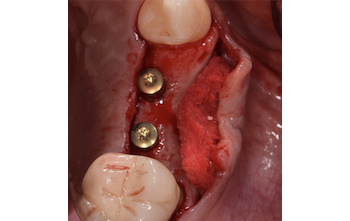
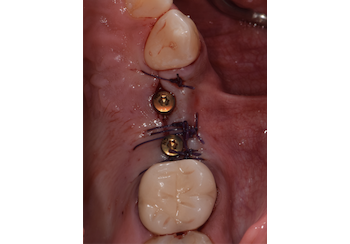
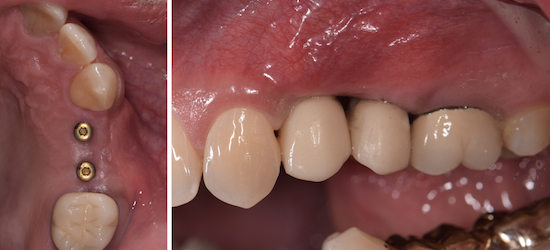
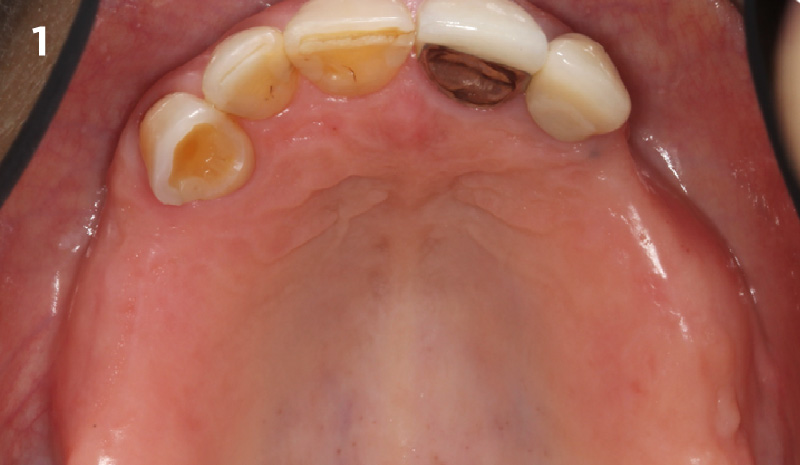
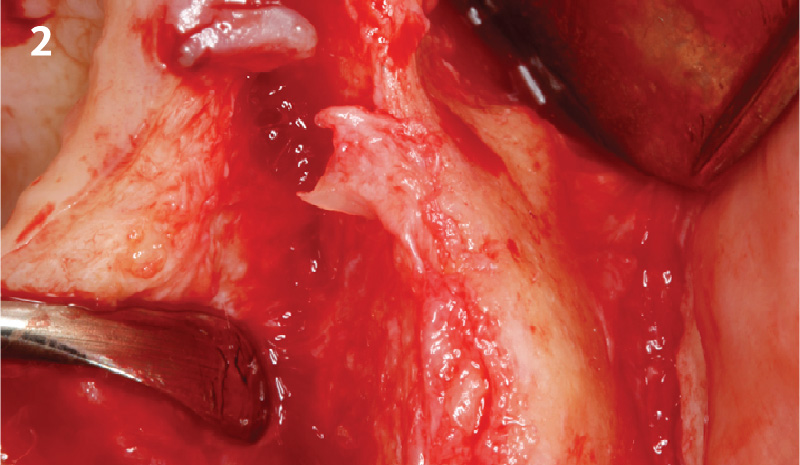
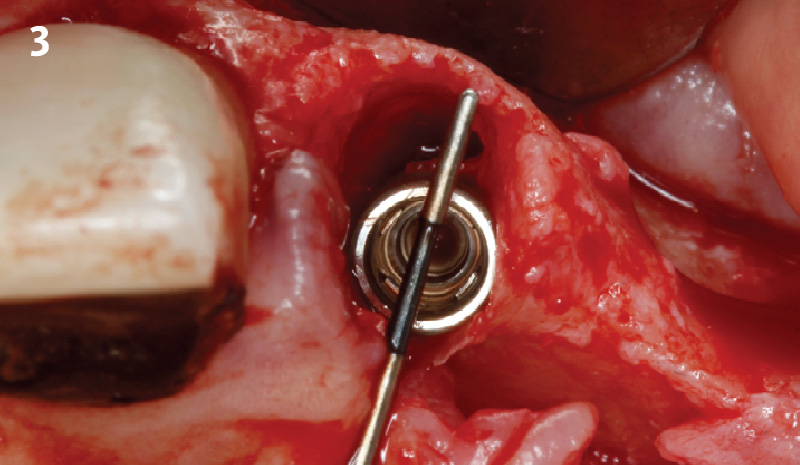
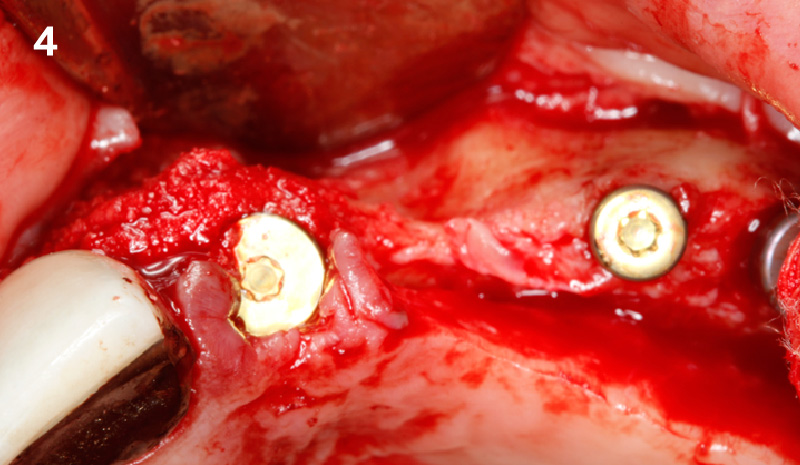
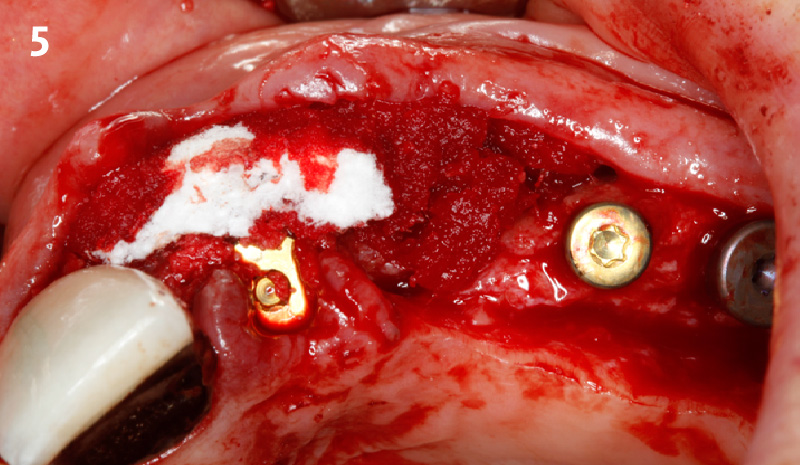
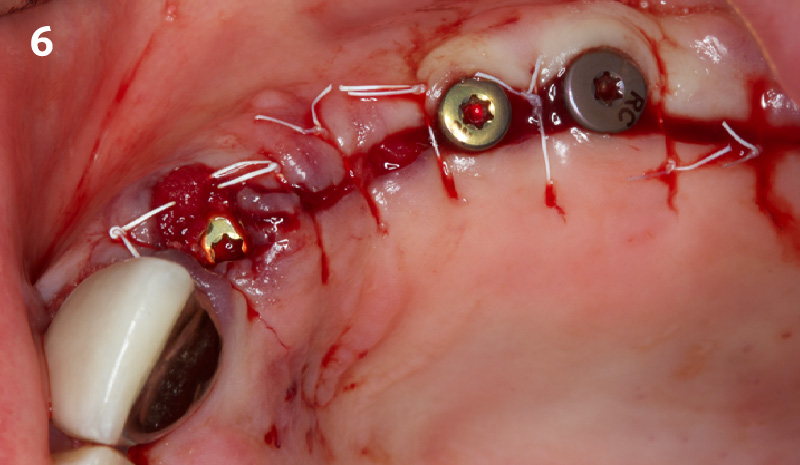
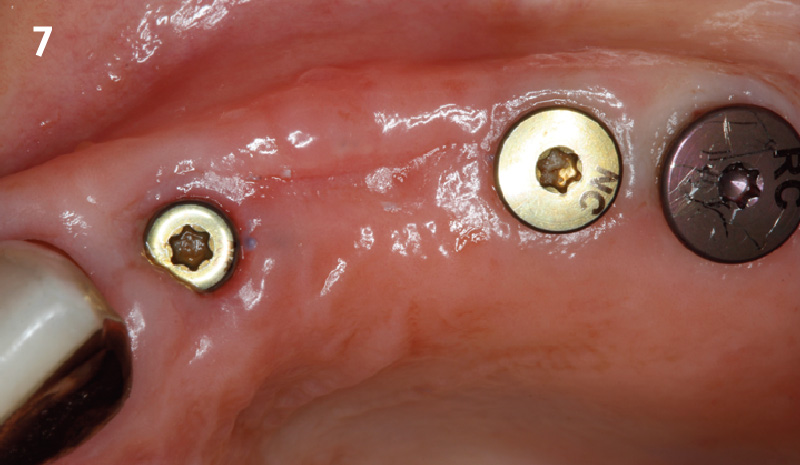
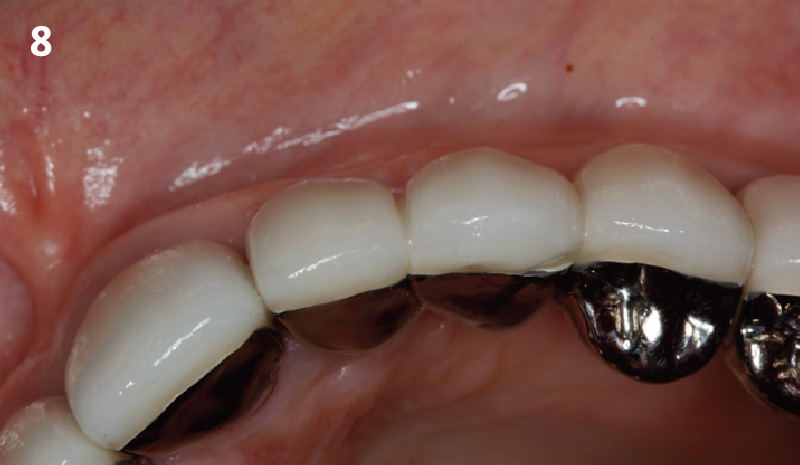
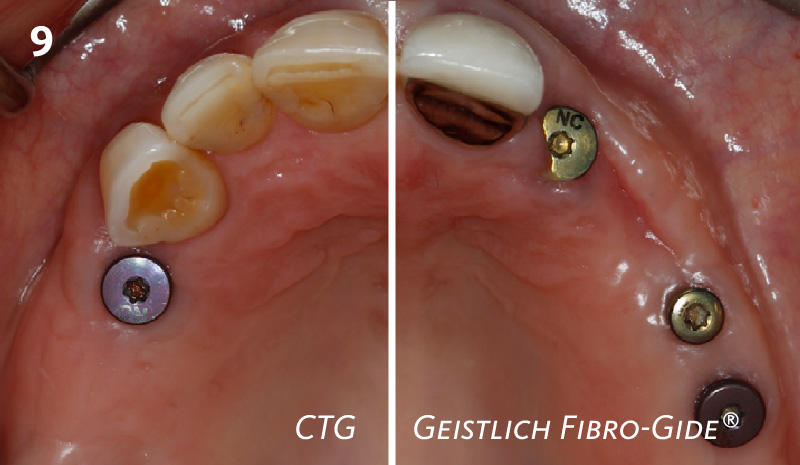
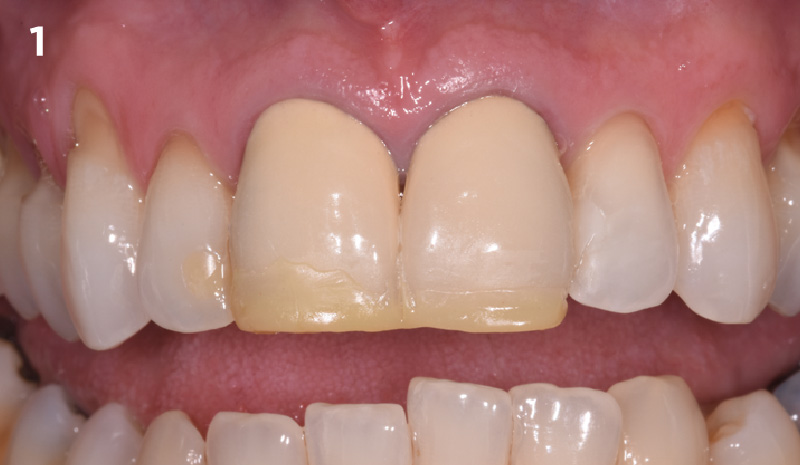
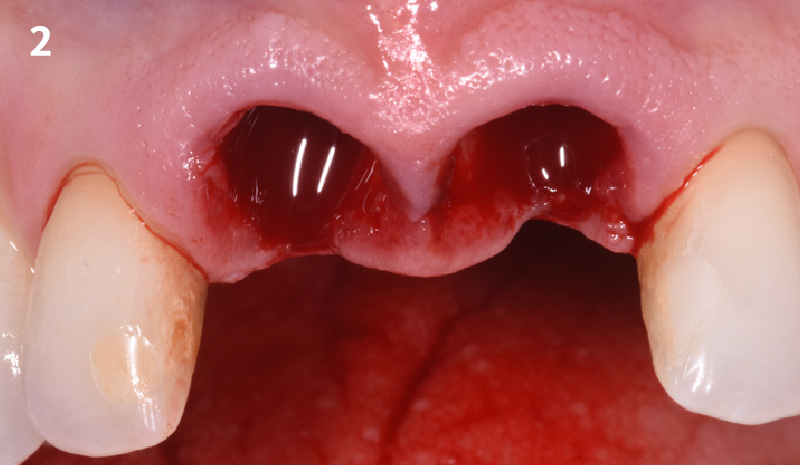
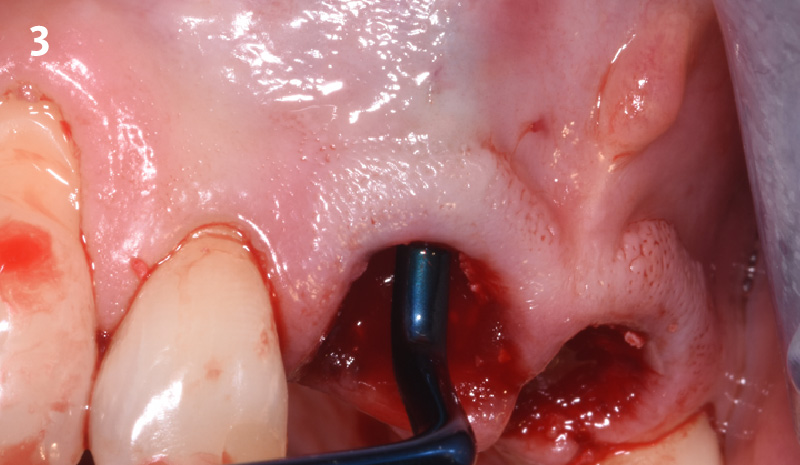
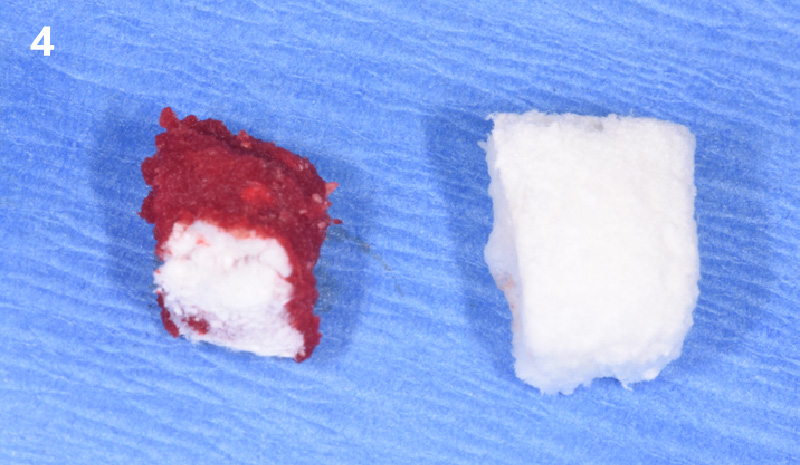
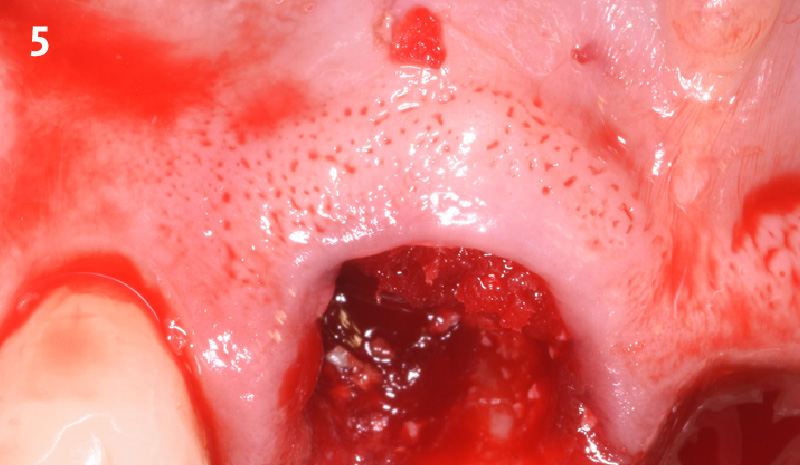
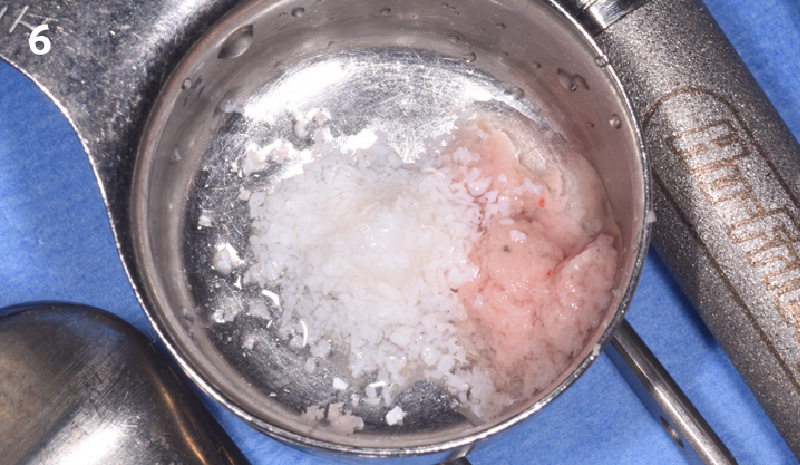
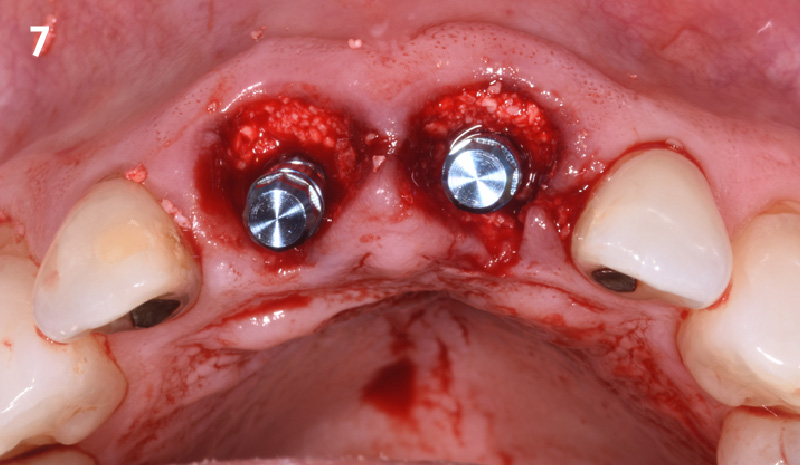
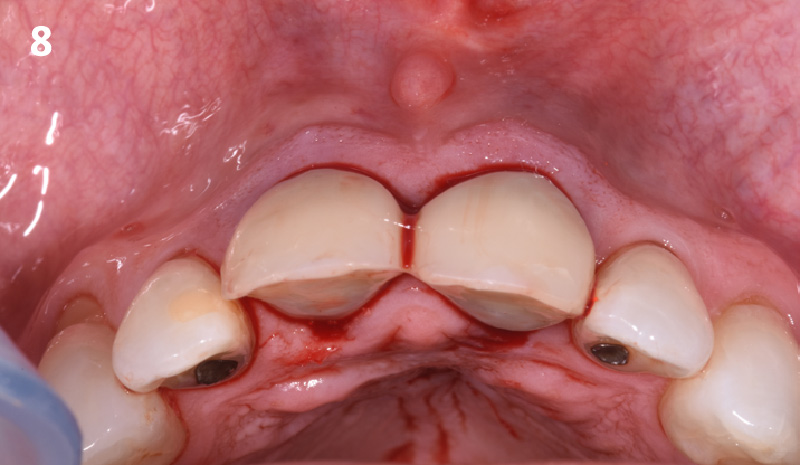
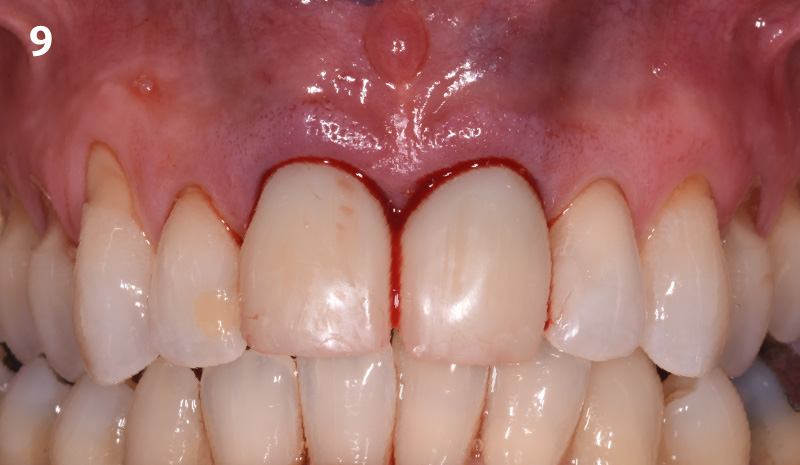
Overview: An 18-year-old AA male presented with trauma to the anterior maxilla. Teeth #7,8,9 were exfoliated and #10 was impacted. Recommended treatment included extraction #10 with major GBR using Geistlich vallomix™ for future dental implant FPD #7-10. Full thickness flap reflection with verticals #6&11 was performed. Tooth #10 was extracted, and area debrided. Ridge augmentation was performed using Geistlich Bio-Gide® membrane and Geistlich vallomix™ bone graft, then primary closure achieved. Healing at 2,4, and 6 weeks showed proper ridge dimensions.