
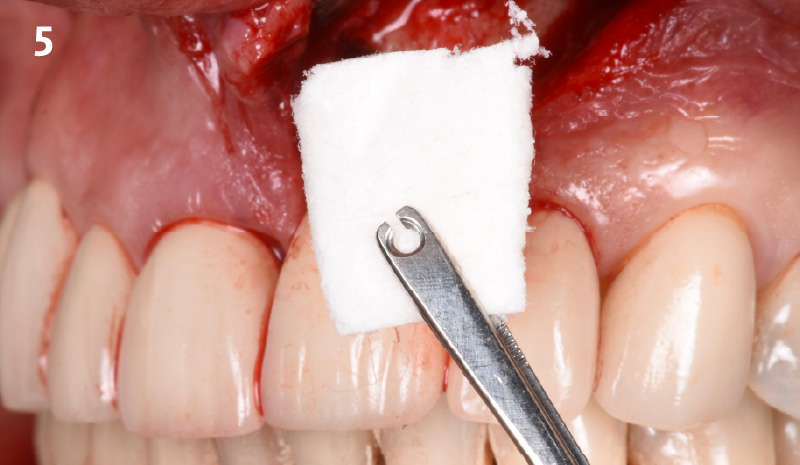
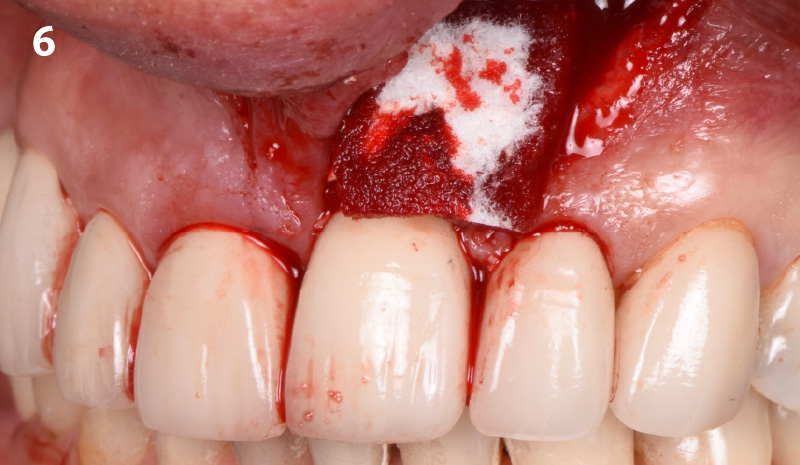
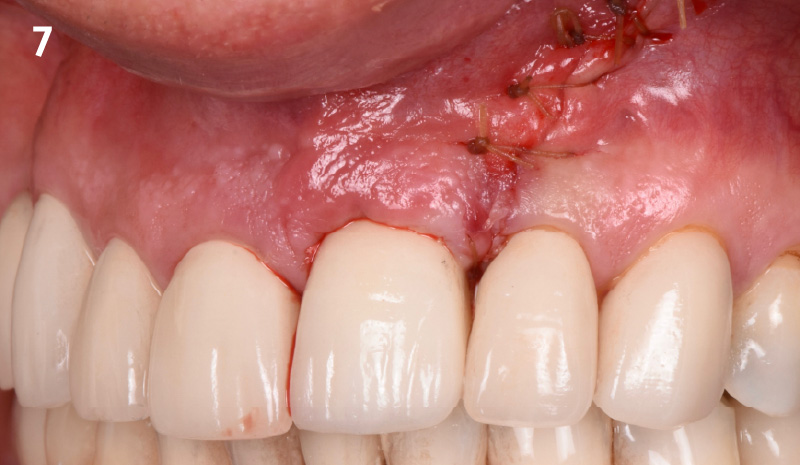
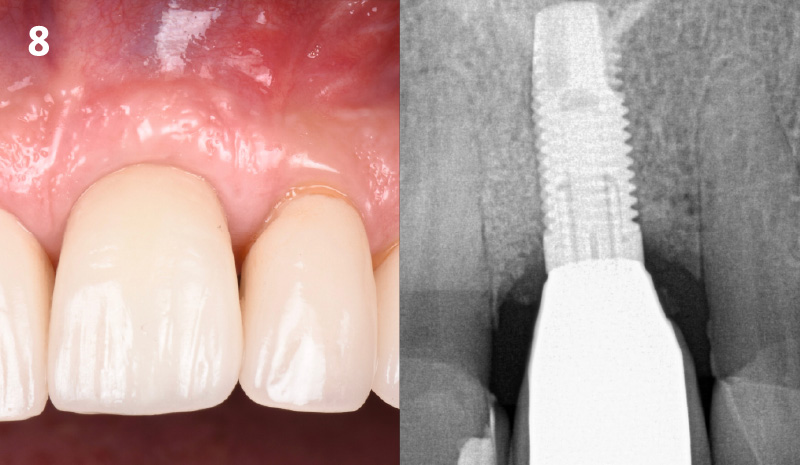
CLINICAL CASE
Mix & Match! Buy ANY 10 Biomaterial Products, Get 3 Free! Use code Q2BG10 Learn More
GEM 21S®, the first recombinant growth factor product for use in oral regenerative surgery. Learn More









