What is the difference between Geistlich Bio-Gide® Forte and Geistlich Bio-Gide®?
Geistlich Bio-Gide® Forte is based on the proven Bio-Gide® technology, with higher stability and alternative handling. Geistlich Bio-Gide® Forte can be easily unfolded and repositioned when applied to the defect site while still being easily adaptable to the underlying ridge contour.1 Geistlich Bio-Gide® Forte can be trimmed in its hydrated state.1 These user-friendly handling properties support surgical efficiency and provide flexibility during application.
- Data on File, Usability Test 2025, Geistlich Pharma AG, Wolhusen, Switzerland.
How is Geistlich Bio-Gide® Forte Processed?
Geistlich Bio-Gide® Forte is processed with the same proven Bio-Gide® technology, also used in Geistlich Bio-Gide® and Geistlich Bio-Gide® Compressed. It undergoes the same compression process as Bio-Gide® Compressed, followed by an additional process step that increases membrane stability.1 This extra step is a purely physical treatment, involves no chemical additives and preserves the original composition of the product.
- Data on File, Usability Test 2025, Geistlich Pharma AG, Wolhusen, Switzerland.
Does the extra processing step for Geistlich Bio-Gide® Forte affect wound healing?
No, Geistlich Bio-Gide® Forte does not negatively impact the wound healing process. It is a native collagen membrane without further cross-linking or chemical additives and is based on the proven Geistlich Bio-Gide® technology. This technology is recognized for its optimal tissue integration and wound stabilization. 1
- Rothamel D et al. Clin. Oral Implants Res. 2005; 16(3): 369-378*
*This is an animal study and may not be indicative of clinical outcomes.
Does Geistlich Bio-Gide® Forte need to be stabilized?
As Geistlich Bio-Gide® Forte adheres very well to the defect, it is normally not necessary to stabilize with screws, pins or sutures. However, stabilization with screws, pins or sutures is possible due to its high tensile strength and may be indicated to avoid displacement due to shear loading or mobilization.1,2
- Data on File, Design Verification Test AB-P307-JB-192, Geistlich Pharma AG, Wolhusen, Switzerland.
- Geistlich Bio-Gide Forte IFU. Rev. 2025-02
What happens if the Geistlich Bio-Gide® Forte membrane is applied with the rough surface towards the soft tissue?
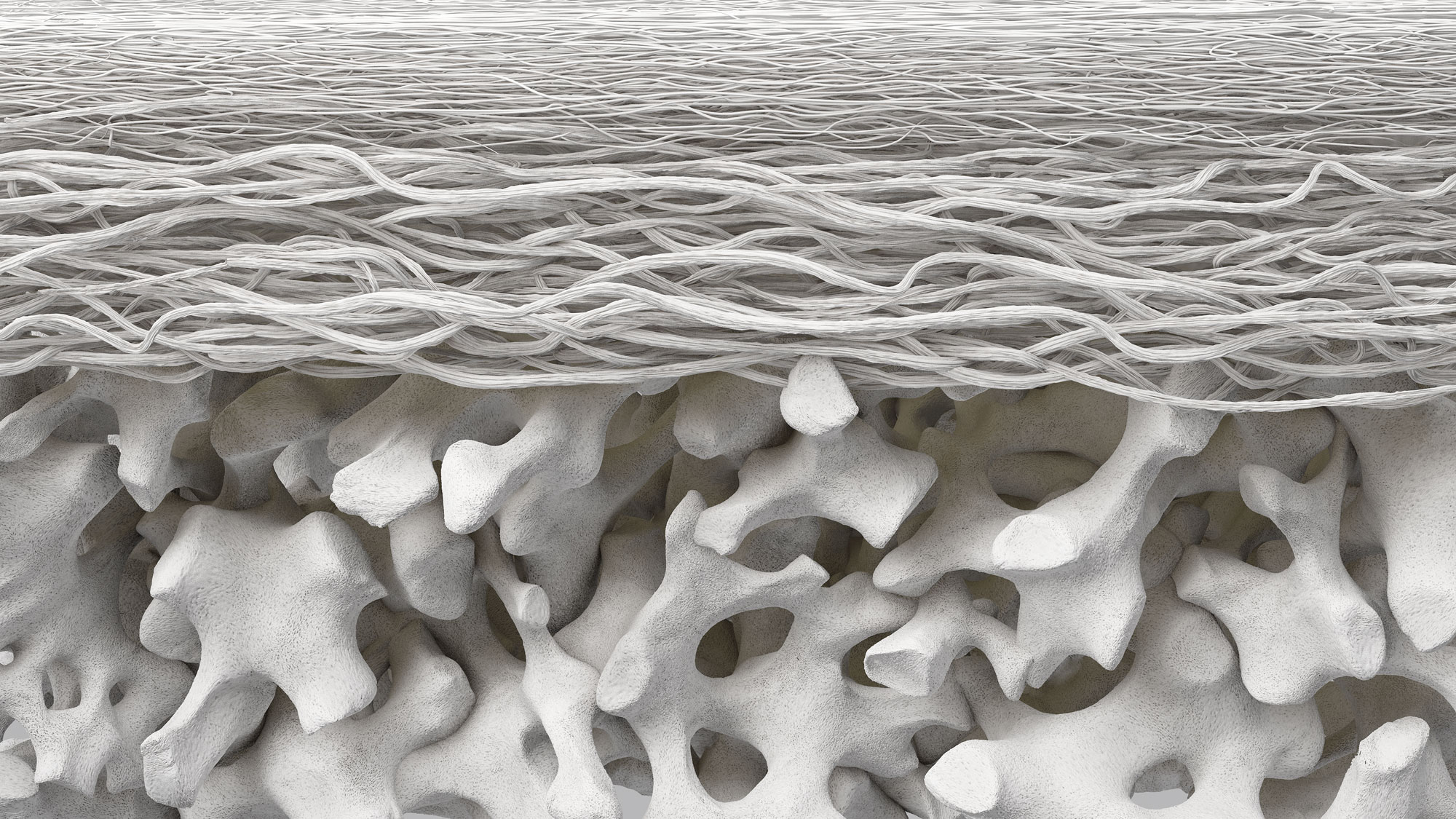
The bilayer structure of Geistlich Bio-Gide® Forte has a compact and porous surface. The compact layer of Geistlich Bio-Gide® Forte exhibits higher cell occlusiveness than the rough, porous layer, preventing the downgrowth of epithelial cells. If the membrane is applied with the porous surface placed toward the soft tissue, integration of the bone cells may take place more slowly. However, it is not necessary to remove the membrane.
Is there cross-linking added to Geistlich Bio-Gide® Forte and what is the difference to “chemically” cross linked?
Geistlich Bio-Gide® Forte is a native collagen membrane without further cross-linking or chemical additives. Studies have shown that cross-linking of bovine and porcine-derived collagen membranes can reduce tissue integration, delay vascularization, and increase the risk of complications like foreign body reactions and dehiscence.1,2
- Rothamel D et al. Clin. Oral Implants Res. 2005; 16(3): 369-378*
*This is an animal study and may not be indicative of clinical outcomes.
- Tal H, et al.: Clin Oral Ipl Res 2008; 19: 295-302
Can Geistlich Bio-Gide® Forte be used in Open Healing?
Geistlich Bio-Gide® Forte can be used submerged or in an open healing situation depending on the surgeon’s preference.1 The advantages of the secondary intention healing of Geistlich Bio-Gide® include the ability to perform a flapless surgery and the preservation of the mucogingival line.2
- Geistlich Bio-Gide Forte IFU. Rev. 2025-02
- Cardaropoli D, et al.: Int J Periodontics Restorative Dent 2012, 32(4): 421-30.
What is the barrier time for Geistlich Bio-Gide® technology?
Expert oral surgeons have estimated that a membrane used in Guided Bone Regeneration should maintain its barrier function until the provisional matrix and woven bone are present. Therefore, barrier duration is considered to be necessary for 4 to 6 weeks, in most cases. Geistlich Bio-Gide® technology has proven to support bone regeneration on an equivalent level as membranes with a longer barrier function, with the additional benefit of complication-free wound healing.1-4
- Tal H, et al. Clin Oral Impl Res 2008; 19: 295-302.
- Becker J, et al. Clin Oral Impl Res 2009; 20(7): 742-49.
- Schwarz F, et al. Clin Oral Impl Res 2008; 19(4): 402-15.*
*This is an animal study and may not be indicative of clinical outcomes.
- Nahles S, et al. Clin Oral Implants Res. 2013 Jul;24(7):812-9.”















 Stiffness in Hydrated State:
Stiffness in Hydrated State: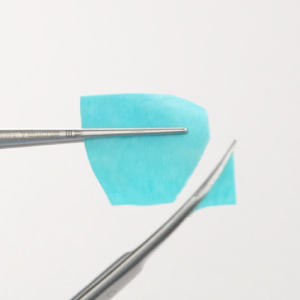 Cutting in dry and hydrated state:
Cutting in dry and hydrated state: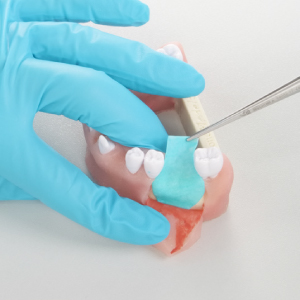 Easy reposition:
Easy reposition: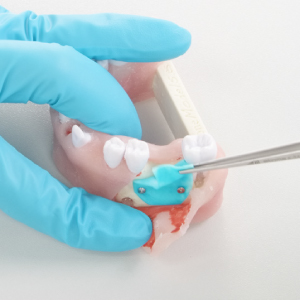 Easy Unfolding:
Easy Unfolding:




