The Original Xenograft Bone Substitute
Still Exactly Like No Other

PRODUCT INFORMATION
About Geistlich Bio-Oss®
The leading xenogeneic bone substitute in regenerative dentistry worldwide.1,2
- The osteo-conductive properties lead to effective and predictable bone regeneration3‑5
- Easy to apply and can be used in a variety of therapeutic areas
- Documented in more than 1300 publications
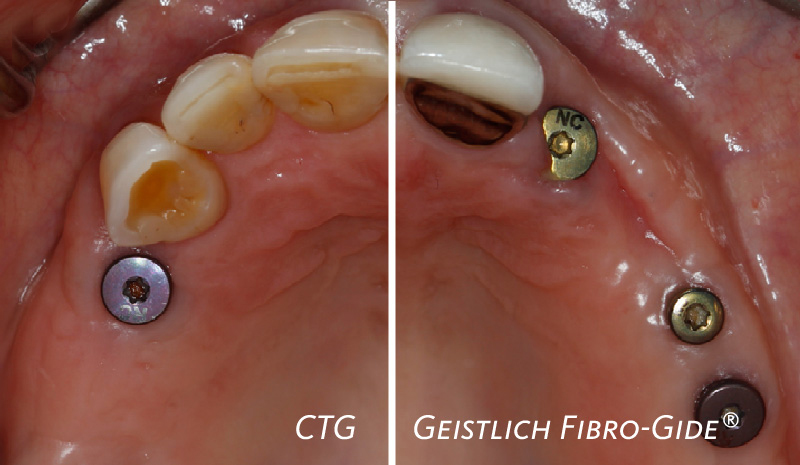
- Long-term data demonstrates predictable results in Guided Bone Regeneration treatments used in combination with Geistlich Bio-Gide®6
UNIQUE PROPERTIES
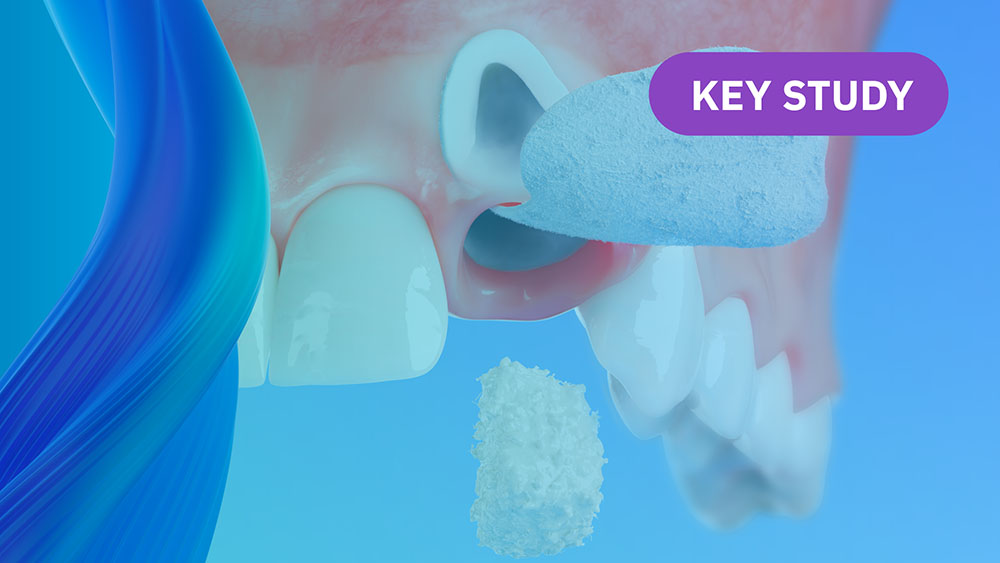
Geistlich Bio-Oss® becomes fully integrated into living bone over time to maintain space and preserve regenerative volume7,8
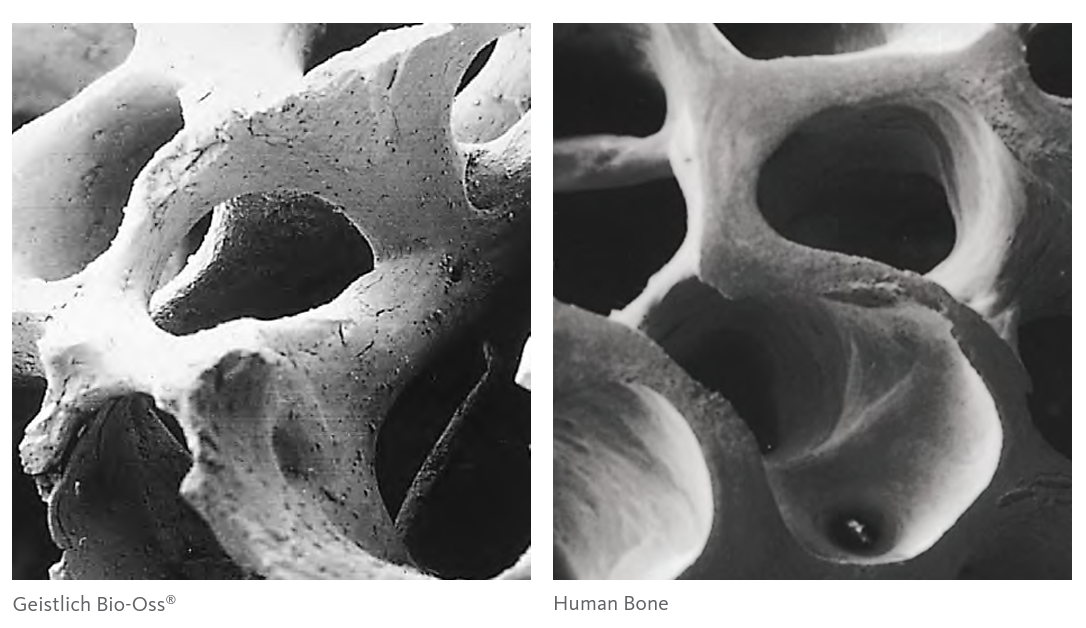
In the production of Geistlich Bio-Oss®, derived from bovine bone, these complex tissues are reduced to their essential form. The native crystalline structure, which is highly similar to human bone, is preserved through our unique, patented technology.
HANDLING & EASE OF USE
Geistlich Bio-Oss® has excellent handling properties and provides predictable results in daily clinical use
- Hydrophilic properties promote rapid and complete hydration
- Easily modeled and adheres optimally to the walls of the defect
- Subsequent application of Geistlich Bio-Gide®, a collagen membrane, enables undisturbed healing which leads to new bone regeneration
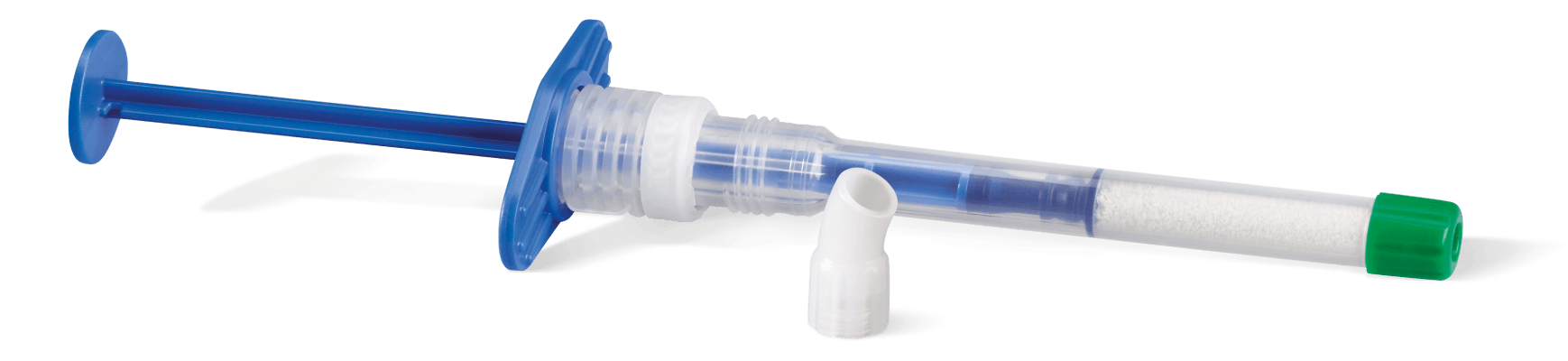
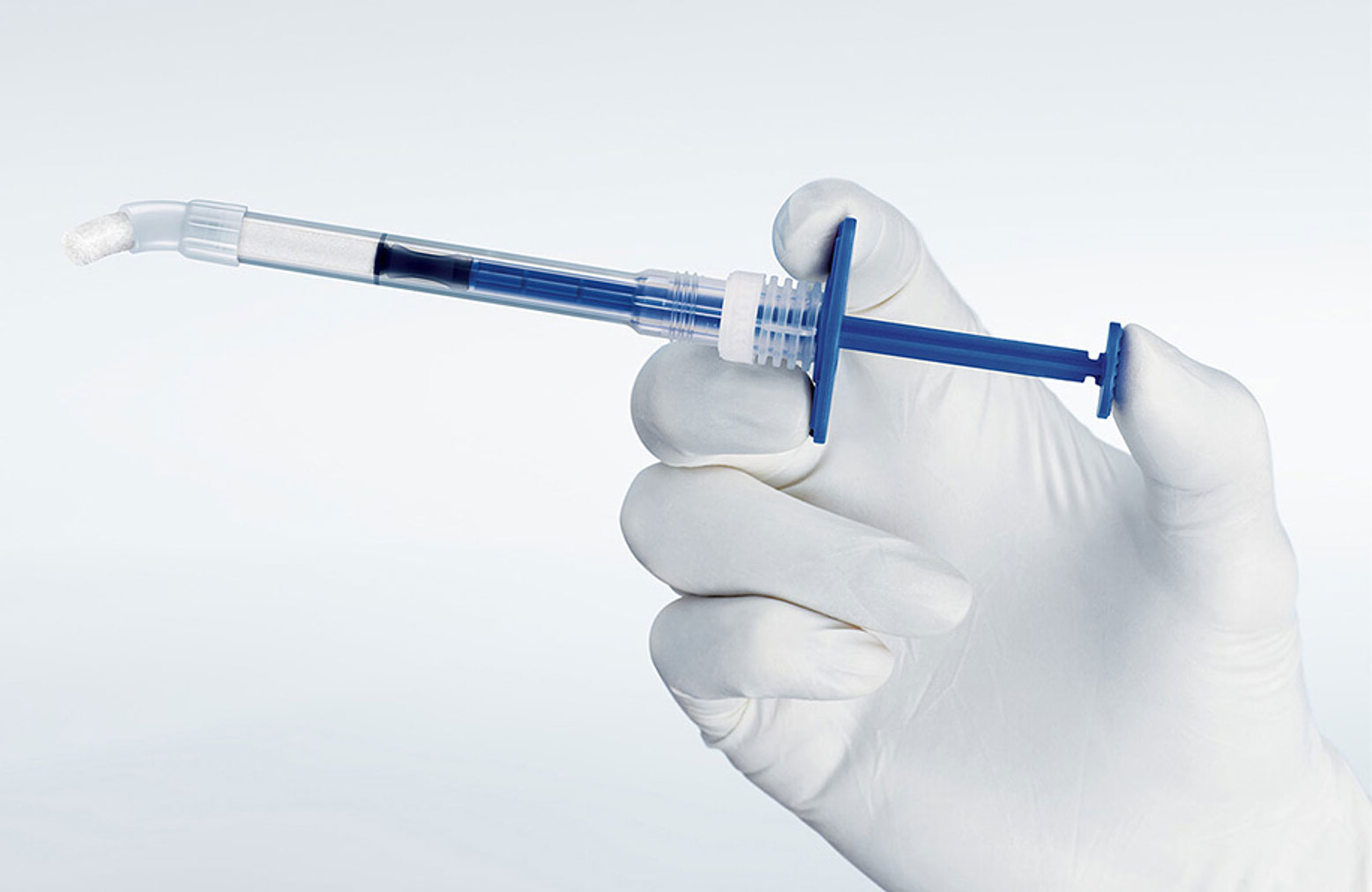
Handling Geistlich Bio-Oss®

Treatment made simple
A pre-filled syringe containing Geistlich Bio-Oss® granules
- Easy-to-use applicator for faster application, precision and convenience
- Greater flexibility in a variety of clinical situations
- Available in both large and small particles
- Optimal access that allows easy placement into posterior defects
Related Content
Geistlich Bio-Oss®
Geistlich Bio-Oss Pen®
Frequently Asked Questions
- iData Research Inc., US Dental Bone Graft Substitutes and other Biomaterials Market, 2022.
- iData Inc., European Dental Bone Graft Substitutes and other Biomaterials Market, 2015.
- Orsini G, et al.: J Biomed Mater Res, B: Appl Biomater 2005;74(1): 448-57.
- Jung RE, et al.: Clin Oral Implants Res 2013; 24(10): 1065-73.
- Aghaloo TL, Moy PK.: Int J Oral Maxillofac Implants 2007; 22 Suppl: 49-70.
- Jung RE, et al.: Clin Oral Implants Res. 2013; 24(10):1065-73.
- Galindo-Moreno, P. et al. (2014). Clin Oral Implants Res.25(3):366-71.
- Araújo, MG. et al. (2010). Clin Oral Implants Res. 21(1):55-64.