Which data is needed for the planning?
For the planning and production of the grid structure, data must be supplied in DICOM format. DICOM means Digital Imaging and Communications in Medicine. This format is standardized and used in archiving and exchange of information in medical image data management. This allows data transfer (e.g. digital images) between systems of various manufacturers.
The DICOM format provides us the data of your patients that we need for the planning and production of the grid structure. Digital images you have created with your CT or CBCT of the defect region must be saved in DICOM format (.dcm), then sendt to us.
The STL interface (Surface Tessellation Language, a method to describe surfaces with triangles or Standard Triangulation Language) is a (quasi-) standard interface of many CAD systems.
This data interface is typically used to send geometric information of three-dimensional data models for production using generative production methods or rapid prototype facilities.
If you have your images made by a radiologist, please ask that the data be saved in DICOM format.
What is a titanium/titan mesh?
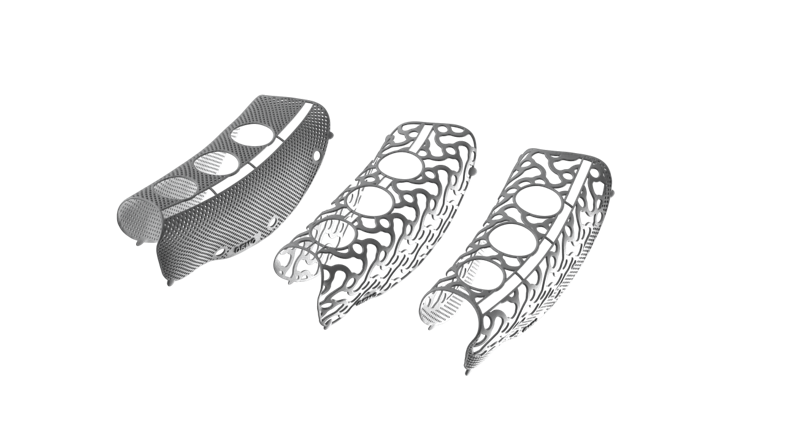
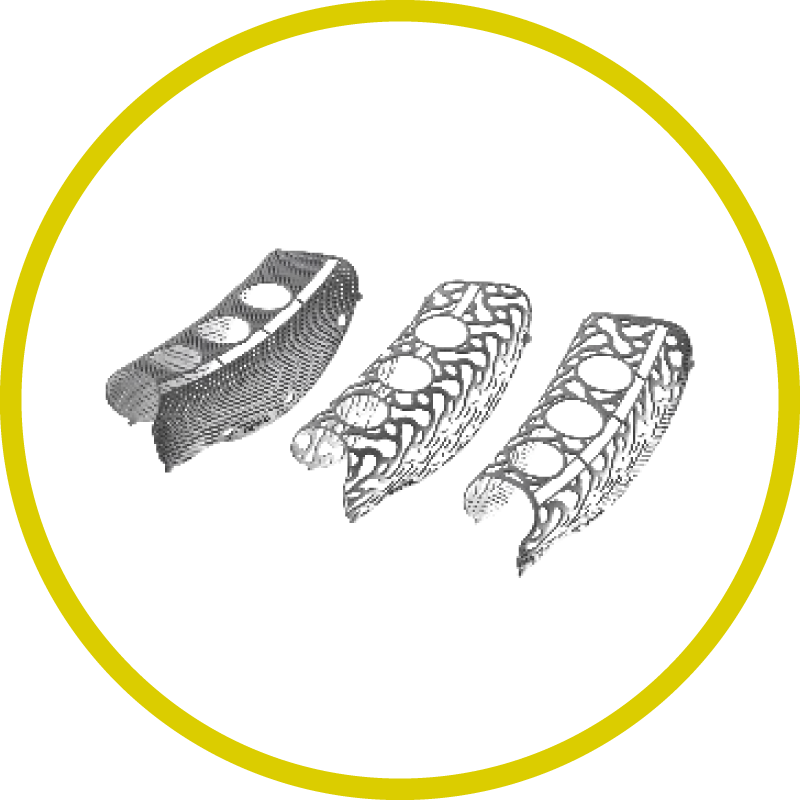
Yxoss CBR® is produced using pure titanium or titanium alloys (medical grade 1) certified for medical use. These materials fulfill the requirements of ISO 5832/2 and ISO 5832/3 for titanium and titanium alloys, respectively. These materials are biocompatible, corrosion resistant, non-toxic in biological environments and allow for distortion-free radiologic imaging. Also, they have a high specific strength that makes possible even with a lean material thickness, a volume stable, protected space for bone regeneration.
When should Yxoss CBR® be used in bone augmentation?
In the framework of “backward planning” the implant should always be inserted at the prosthetically correct position. If a bone deficit or defect is in this location, Yxoss CBR® can be utilized as space holder during the bone regeneration similar to a furring construct. This may be the case for immediate or delayed augmentation in extraction alveoli, for a larger reconstruction of the alveolar process, or generally to fill other maxillofacial defects.
The principle of individual, customized space holders requires the use of volume-filling material such as autologous bone or/and bone substitute material. This makes a three-dimensional augmentation in accordance with the individual requirements of the patient possible.
Generally, the use of membranes or meshes, especially in the fields of implantology and regenerative periodontal surgery, is today standard procedure. Typically, membranes are always utilized in oral surgery or maxillofacial surgery if a bone defect or bone resorption is present and new bone needs to be built up, or to support bone regeneration.
Does Yxoss CBR® need to be sterilized before use?
The usual hygiene requirements for oral surgery and implantology also apply for the use of Yxoss CBR®.
Yxoss CBR® is delivered non-sterile in double-peel bags and needs to be sterilized before using it on the patient surgically. Remove the double sealed bags from the carton and transfer directly for sterilization to the autoclave. The implant should be autoclaved at 135°C for 20 minutes in confined water vapor.
The sterile titanium scaffold can then be taken out of the sterilization bag and used immediately for surgery.
Can Yxoss CBR® be re-sterilized?
No, Yxoss CBR® is an individually produced product for single use in a patient. A second autoclaving or hot air sterilization cannot restore the physical properties of the grid after using the product on the patient. Also deformations of the grid can appear during re-sterilization and the patient fit cannot be guaranteed. Hence, Yxoss CBR® can only be used once. In case of re-sterilization, ReOss® does not except any liabilities, as the product is not certified to be used after re-sterilization.
How is Yxoss CBR® different from other titanium grids for bone augmentation?
Yxoss CBR® is an individually produced titanium grid for three dimensional bone augmentation designed for a single patient. Hence, surgery time is significantly reduced. The edges and surfaces are designed to be tissue-friendly. Other titanium grids require a chairside complex contouring of the grid. The space holder function of the mesh allows for a vertical augmentation of the bone deficit. Thanks to the Easy Removal function (see below), the subsequent removal of the mesh is without complications and not associated with bone loss.
How does Yxoss CBR® differ from resorbable membranes?
Being resorbable describes the property of a membrane to be biodegradable by physiological processes in the body of the recipient. Non-resorbable membranes or meshes are bio-inert, can remain over a longer period of time in the planned location, and enable bone augmentation. The advantage of an unlimited barrier time and related exclusion of soft-tissue from the region of bone regeneration as well as 3D stability with Yxoss CBR® allows for the undisturbed three-dimensional creation of bone.
Which criteria are considered in the production of Yxoss CBR® individual products?
The careful selection of raw materials, according to the given ISO standards, and strict control and certification of processes in the production of ReOss®products meet the highest quality and safety requirements. The production process is regularly verified by independent institutions and authorities and a well-documented quality system is in place.
How must Yxoss CBR® be stored?
The system components must be carefully handled and stored in a dry place. This is particularly valid for the custom-made meshes. These should not be subjected to any mechanical stress. Damage or scratches on the surface of Yxoss CBR® can significantly affect the stability and fatigue resistance.
How is Yxoss CBR® applied?
Yxoss CBR® was designed in accordance with the general principles established for the membrane or mesh application. This includes compliance with the biological limits (e.g. distance to the adjacent teeth) according to the current guidelines and the passive, stress-free fit of the mesh. In general, a covered healing of Yxoss CBR® is recommended to avoid a possible bacterial colonization of the augmented area and thus a loss of the augmentation.
Must Yxoss CBR® be additionally fixed/secured?
For the perfect fixation of Yxoss CBR®, a fixation system with diameters from 1.3 to 1.5 mm (in the threaded region) is recommended. Due to the exact fit of Yxoss CBR®, fixation with one screw is often sufficient for smaller defects. In the treatment of larger defects with Yxoss CBR® more than one fixation screw is recommended.
Due to the patent protected texture of Yxoss CBR®, variable placement of the fixation screw is possible. The meshes are so stably in place during bone healing that, according to the principle of mechanical rest, optimal conditions are present for bone regeneration and de novo bone synthesis to enable the desired new bone contouring.
Can Yxoss CBR® be used without membrane?
Yxoss CBR® is a titanium grid that does not replace the function of a membrane. According to the principles of guided bone regeneration, Yxoss CBR® should be used in combination with a protective membrane. Current clinical data are based on the combined use of Yxoss CBR® with a collagen membrane, for example, Geistlich Bio-Gide®.
Should augmentations under Yxoss CBR® be mixed with antibiotics?
Therapy with Yxoss CBR® should proceed with the same intra- and post-operative regimen that is typically used for augmentation procedures for implant therapy. There is no product-related need for antibiotic protection. In the case of bone augmentation with autogenous bone and in orthognathic surgery, it has been shown in prospective, randomized studies that a pre-operative single-dose systemic administration of antibiotics is indicated to prevent postoperative infections. When using bone substitute materials for augmentation, antibiotic prophylaxis is also recommended, although systematic studies are lacking.
How long should a Yxoss® mesh remain implanted during guided bone regeneration before removal?
A barrier function is only required as long as different types of tissue compete for dominance in the healing defect and sufficient stability of the augmented bone to accommodate implants has been reached. Following the ITI classification of delayed implantation (Type 3), the mesh can be removed after aprox. 16 weeks. However, a case-based analysis considering the augmenting volume is always recommended. Especially with complex defects, longer healing periods should be considered.
Have inflammatory reactions been associated with Yxoss CBR®?
The most common complications after oral surgery or oral-maxillofacial surgery include postoperative infections. Here, the usual treatment recommendations apply. In generally, however, an inflammation can be a natural process in the course of wound healing (inflammatory cascade in the course of secondary wound healing). Yxoss CBR® is biocompatible. Product-related reactions have not been observed and are therefore not expected. Infections due to inadequate hygiene (especially in the oral cavity) can negatively affect the healing process.
Is it possible to have an allergic reaction to Yxoss CBR®?
Since Yxoss CBR® is made of titanium (Medical Grade 1), allergic reactions and inflammatory tissue reactions can be almost completely excluded. In patients with a known titanium allergy the use is contraindicated.
What evidence exists describing the effectiveness of Yxoss CBR®?
Titanium meshes have been used in bone regeneration for many decades and have been scientifically positively evaluated in many publications.
What happens to the Yxoss® titanium mesh after implantation?
Disposal of the Yxoss CBR® is to follow RKI-guidelines. Secondary use is not warranted.
What must be considered when removing Yxoss CBR®?
With respect to the chosen design, our technicians include an area in the crestal region of the implant to allow atraumatic removal without having to re-form a full flap. This allows easy removal, even if the newly formed bone should have penetrated the grid structure. To remove the fixation screws, turn with the screwdriver and pull out with an appropriate tool or by hand. Utilizing the Easy Removal function, a light lever action is performed at the appropriate interface to remove Yxoss CBR® in at least two parts.
Can Yxoss CBR® be applied incorrectly?
Normally Yxoss CBR® is designed so that it only fits in one position precisely. This will be confirmed intraoperatively by each surgeon. If Yxoss CBR® is used and fixed in position other than designed, bone regeneration may take place more slowly than expected and might not adopt the desired shape of the alveolar process. The barrier function can still be realized.
How is the bone for filling Yxoss CBR® harvested?
Autogenous bone is derived primarily from intraoral donor sites, located in accordance with usual sites. These are: locally at the site of implantation, from the region of linea obliqua, retromolar, the chin area or the spina nasalis, or from the tuberosity. For very large defects, harvesting of iliac crest spongiosa may also be required. Bone material must be loaded into the mesh in particle form. The use of filtered bone is not recommended.
When is bone augmentation necessary?
Bone loss the jaw region, compared to the rest of the body, is a very common process as a consequence of single or multiple tooth loss. Likewise, chronic inflammation from periodontitis or around tooth roots as well as cysts or tumors may lead to degradation processes resulting in bone deficits. If an implant is inserted for dentures, to replace a tooth, or for other functional reasons, a reconstruction of the bone may be necessary in order to create new vertical and horizontal dimensions.
What happens after a tooth extraction?
After tooth extraction, the bone resorbs in the following months and years. After one year, up to 50% of the jaw width and up to 2 mm jaw height can be lost. This leads to impaired aesthetics, decreased masticatory function with possible resulting joint problems, as well as adverse effects on the articulation due to the changes in the interior of the mouth.
What are the causes of bone loss?
Bone loss in the jaw region, compared to the rest of the body, is a very common process as a consequence of single or multiple tooth loss. Likewise, chronic inflammation from periodontitis or around tooth roots as well as cysts or tumors may lead to degradation processes resulting in bone deficits.
How do I access the IFUs for Geistlich products?
The IFUs for Geistlich products can be found using this page.